新型菌株 Lichtheimia sp. UV-16 产生的外聚半乳糖脲酶及其水解特性
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
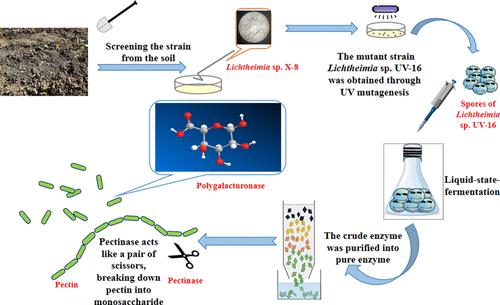
首次从土壤中分离出产果胶酶的菌株 Lichtheimia sp.随后,通过紫外诱变获得了果胶酶活性提高了 1.23 倍的 Lichtheimia sp. UV-16,并优化了其液体发酵工艺,使果胶酶活性从 455.6 ± 12.7 U/mL提高到 3202.0 ± 82.1 U/mL。粗酶经盐析和阴离子交换树脂纯化,纯化率为 2.28 倍,产率为 36.5%。纯酶的最佳反应温度为 60 ℃,最佳 pH 值为 5.5。薄层色谱法(TLC)和高效液相色谱法(HPLC)证实该酶是一种外聚半乳糖醛酸酶,果胶水解效率超过 99%。此外,在果汁浆中加入纯酶可大大提高果汁产量,因此这种聚半乳糖醛酸酶在饮料行业的应用前景广阔。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exopolygalacturonase Production from the Novel Strain Lichtheimia sp. UV-16 and Enzyme Hydrolysis Properties
A pectinase-producing strain, Lichtheimia sp. X-8, was isolated from the soil for the first time. Subsequently, Lichtheimia sp. UV-16, with a 1.23-fold increase in pectinase activity, was obtained via UV mutagenesis, and optimization of its liquid fermentation process boosted pectinase activity from 455.6 ± 12.7 to 3202.0 ± 82.1 U/mL. The crude enzyme was purified by salting out and anion exchange resin, with a purification ratio of 2.28-fold and a yield of 36.5%. The optimal reaction temperature for the pure enzyme was 60 °C with an optimal pH of 5.5. Thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) confirmed that the enzyme was an exopolygalacturonase, achieving over 99% efficiency in pectin hydrolysis. Furthermore, incorporating pure enzymes into juice pulps can substantially enhance the juice yield, which makes this polygalacturonase a promising application in the beverage industry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


