通过移ph处理改变蛋黄蛋白结构,实现界面重组;改善溶解度,增强油水界面吸附和乳化性能
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
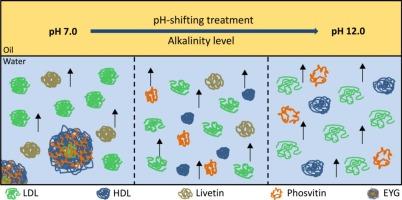
本研究探讨了不同碱度的pH值转换处理对蛋黄蛋白(EYP)乳化的影响,并研究了油水界面吸附的基本机制。pH值偏移处理的碱性增加会暴露蛋黄蛋白中更多的疏水基团,从而改变其三级结构。此外,pH 值偏移处理降低了溶液粒度(P < 0.05),这可能是通过将不溶性蛋黄颗粒(EYG)分解成更小的亚单位。在 pH 值为 12.0 的条件下,蛋黄(EY)溶液的浑浊度最小,溶解度最大(81.62%)。在初始吸附过程中,pH 值为 9.0 的移位溶液表现出最大扩散率(0.049 mN/m/s),与最小溶液粒度(88.36 nm)相关。随后,碱性 pH 值偏移诱导蛋白质在油水界面重新排列,导致在 pH 值为 12.0 的偏移条件下产生最大界面压力(21.01 mN/m)和粘弹性模量(44.55 mN/m)。这使乳液稳定性提高了 23.82%,起泡指数最低(21.82%)。这些发现对于提高 EYP 的利用率和推广 EY 作为食品乳化剂至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Disturbing egg yolk protein structure via pH-shifting treatment for interface reorganization: Improving solubility to enhance oil-water interface adsorption and emulsification properties
This study explored the impact of varying alkalinity levels in pH-shifting treatments on egg yolk protein (EYP) emulsification and investigated the underlying oil-water interface adsorption mechanism. Increasing alkaline pH-shifting treatment exposed more hydrophobic groups within EYP, altering its tertiary structure. Moreover, pH-shifting treatment reduced solution particle size (P < 0.05), possibly by disintegrating insoluble egg yolk granules (EYG) into smaller subunits. Under pH 12.0-shifting conditions, egg yolk (EY) solution reached minimum turbidity and maximum solubility (81.62 %). During initial adsorption, pH 9.0-shifting solution exhibited maximum diffusion rate (0.049 mN/m/s), correlated with minimum solution particle size (88.36 nm). Subsequently, alkaline pH-shifting induced protein rearrangement at the oil-water interface, leading to maximum interfacial pressure (21.01 mN/m) and viscoelastic modulus (44.55 mN/m) under pH 12.0-shifting conditions. This increased emulsion stability by 23.82 % with the lowest creaming index (21.82 %). These findings were crucial for enhancing EYP utilization and promoting EY as a food emulsifier.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


