硫介导糖基转移酶UGT76G1与雷鲍迪糖苷D相互作用促进雷鲍迪糖苷M合成
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
雷鲍迪糖苷M (Reb M)是一种高甜度的零热量甜味剂,由于产量低、纯度低,在生产上面临挑战。UGT76G1是一种尿苷二磷酸葡萄糖(UDPG)依赖的糖基转移酶,它与雷鲍迪苷D (Reb D)形成β-1,3-糖苷键生成雷鲍迪苷M,但其效率低于甜菊苷(ST)。本研究鉴定了变异UGT76G1- l200a /L379 M (No.11),其对Reb D的酶活性比野生型UGT76G1 (WT)增加了10倍。与mbSUS相结合,No.11有效地合成了Reb M,在50°C条件下,从34.89 mM Reb D中提取60 min,收率达到96.85%。分子动力学揭示了这种催化活性增强背后的分子机制:与WT相比,No.11、UGT76G1-L200A和UGT76G1-L379 M配合物在Reb d - c19 - glc1 -3-羟基、催化残渣H20和UDPG-C1 '之间的相互作用时间更短、更稳定。均方根波动(RMSF)值和结合自由能分析进一步解释了No.11的优越催化效率。本研究引入了一种新的蛋白质工程方法,通过引入特定的氨基酸来触发非经典相互作用,改善配体与蛋白质的结合和催化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
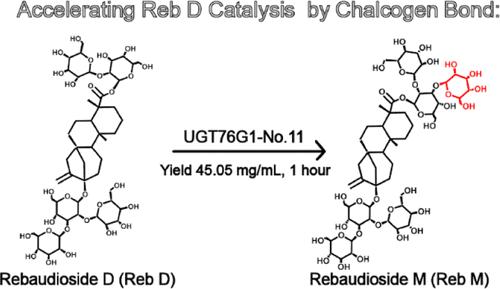
Enhancing Rebaudioside M Synthesis via Introducing Sulfur-Mediated Interactions between Glycosyltransferase UGT76G1 and Rebaudioside D
Rebaudioside M (Reb M), a zero-calorie sweetener with high sweetness, faces production challenges due to its low yield and purity. UGT76G1, a uridine diphosphate glucose (UDPG)-dependent glycosyltransferase, forms a β-1,3-glycosidic bond with rebaudioside D (Reb D) to produce Reb M but with an efficiency lower than that for stevioside (ST). This study identified the variant UGT76G1-L200A/L379 M (No.11), which exhibited a 10-fold increase in enzymatic activity toward Reb D compared to wild-type UGT76G1 (WT). Coupled with mbSUS, the No.11 effectively synthesized Reb M, achieving a 96.85% yield from 34.89 mM Reb D in 60 min at 50 °C. Molecular dynamics revealed the molecular mechanism behind this enhanced catalytic activity: the No.11, UGT76G1-L200A, and UGT76G1-L379 M complexes showed shorter and more stable interactions between Reb D-C19-Glc1-3-hydroxyl, catalytic residue H20, and UDPG-C1’ compared to WT. The root-mean-square fluctuation (RMSF) values and binding free energy analyses further explained the No.11’s superior catalytic efficiency. This study introduces a novel protein engineering approach by introducing specific amino acids to trigger nonclassical interactions, improving ligand–protein binding and catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


