多重共分离/质谱法揭示与T细胞耗竭相关的磷酸酪氨酸信号复合物
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
以抑制受体上调和效应功能丧失为特征的T细胞衰竭在肿瘤免疫逃避中起着至关重要的作用。本研究利用高通量、可重复性和强大的集成离子交换色谱-串联质量标签(IEC-TMT)平台,结合以络合物为中心的定量算法,彻底分析了T细胞衰竭过程中磷酸酪氨酸(pTyr)蛋白复合物的变化。在HeLa细胞生物复制中,定量复合物的相关性为>;0.94,中位变异系数为0.25,证明了该平台的高重复性。这种高通量方法允许在2天内分析312个组分,从T细胞衰竭模型中鉴定出268个pTyr蛋白复合物。对28个复合物的定量分析显示,12个复合物在耗尽的T细胞中表现出显著的丰度变化,特别是影响溶酶体和内质网相关复合物。RTN4是新发现的PPI204蛋白复合物的一个亚基,在耗竭的T细胞中上调。它的下调逆转了T细胞的衰竭,增强了抗肿瘤免疫。这些发现为T细胞耗竭的分子机制提供了新的见解,并提出RTN4作为潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
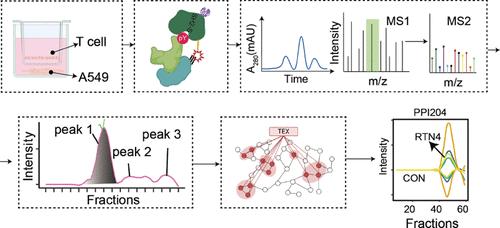
Unraveling of Phosphotyrosine Signaling Complexes Associated with T Cell Exhaustion Using Multiplex Co-Fractionation/Mass Spectrometry
T cell exhaustion, characterized by the upregulation of inhibitory receptors and loss of effector functions, plays a crucial role in tumor immune evasion. This study utilizes a high-throughput, reproducible, and robust integrated ion-exchange chromatography–tandem mass tag (IEC-TMT) platform, coupled with a complex-centric quantification algorithm, to thoroughly profile phosphotyrosine (pTyr) protein complex changes during T cell exhaustion. The platform’s high reproducibility is evidenced by >0.94 correlation and a median coefficient of variation of 0.25 among quantified complexes in HeLa cell biological replicates. This high-throughput approach allowed analysis of 312 fractions within 2 days, identifying 268 pTyr protein complexes from the T cell exhaustion model. Robust quantification of 28 complexes revealed 12 exhibiting significant abundance alterations in exhausted T cells, notably impacting lysosomal and endoplasmic reticulum-associated complexes. RTN4, a subunit of the newly identified PPI204 protein complex, is upregulated in exhausted T cells. Its knockdown reversed T cell exhaustion, enhancing antitumor immunity. These findings provide novel insights into the molecular mechanisms of T cell exhaustion and propose RTN4 as a potential therapeutic target.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


