冷氩等离子体诱导的酪蛋白和肽的聚集性和非聚集性结构变化
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
酪蛋白(CN)是一种常见的过敏原,在现代食品中很难避免。研究了冷氩等离子体(CAP)对CN抗原性降低的影响,重点研究了表位结构和序列的改变。CAP主要含有羟基自由基(∙OH)。经过12分钟的CAP处理,ELISA结果显示抗原性降低80.46 %。透射电镜和电泳显示部分CN聚集,多光谱分析显示部分CN断裂成更小的肽。预测的3D模型表明,位于α-螺旋区的线性表位的破坏可能有助于降低致敏性。将肽序列与免疫信息学方法预测的线性表位进行比较,发现关键过敏序列有所减少或断裂。同时,具有芳香侧链和疏水性基团的氨基酸容易受到cap诱导的修饰。这项研究表明,CAP可能有利于加工低过敏性食品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
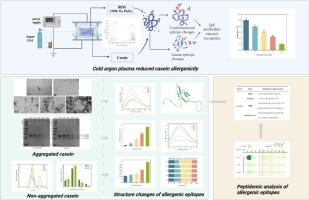
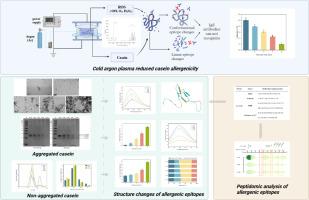
Cold argon plasma-induced aggregated and non-aggregated structural changes in casein and peptidomic insights into allergenicity
Casein (CN) is a common allergen that is challenging to avoid in modern foods. The effect of cold argon plasma (CAP) on reducing CN antigenicity was investigated, focusing on alterations in epitope structure and sequence. CAP mainly contains hydroxyl radicals (∙OH). After a 12-min CAP treatment, the result of ELISA demonstrated an 80.46 % reduction in antigenicity. Transmission electron microscopy and electrophoresis revealed that certain CN aggregated, while multispectral analysis indicated that part of CN was fragmented into smaller peptides. The predictive 3D model suggested the disruption of linear epitopes located in the α-helix region might contribute to the reduced allergenicity. The peptide sequences were compared to the linear epitopes predicted by immunoinformatics approaches, revealing some reduction or breakage of key allergic sequences. Meanwhile, amino acids with aromatic side chains and hydrophobic groups were susceptible to CAP-induced modifications. This investigation demonstrated CAP could be beneficial for processing hypoallergenic foods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


