具有转糖基酶活性的拟杆菌属β-1,3-葡聚糖酶的生化特性
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
β-1,3-葡聚糖酶在功能性低聚糖制备、植物保护和酿酒等领域具有广阔的应用前景。本研究从大盐湖的微生物巨基因组中鉴定出了一个来自拟杆菌属细菌的糖苷水解酶(GH)家族17 β-1,3-葡聚糖酶(BbGlc17A)。BbGlc17A催化层粘连蛋白水解转化为聚合度为3-8的β-糖低聚糖。BbGlc17A的最佳催化条件为pH 6.5和30℃。除了水解活性外,BbGlc17A还表现出转糖苷酶活性,包括催化新的β-1,6-糖苷键的形成。BbGlc17A具有典型的(β/α)8 tim桶状结构和细长的催化槽,与其他典型的β-1,3-葡聚糖酶不同,它促进了转糖苷反应的正向进行。这有效地突出了酶将β-1,3-葡聚糖转化为混合功能低聚糖的潜力。这些结果揭示了GH家族17 β-1,3-葡聚糖酶的催化性质和应用潜力,并为糖活性酶在生物化学中的应用提供了有价值的信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
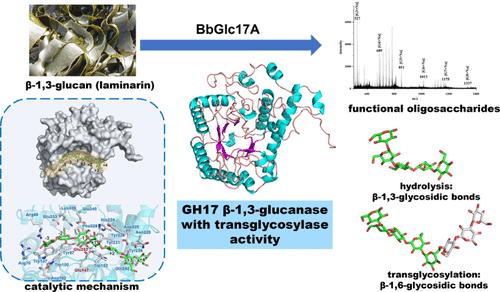
Biochemical Characterization of a β-1,3-Glucanase from Bacteroidetes sp. Having Transglycosylase Activity Suitable to Synthesize β-Glucooligosaccharides
β-1,3-Glucanases have prospective applications in areas such as functional oligosaccharide preparation, plant protection, and breweries. In this study, a glycoside hydrolase (GH) family 17 β-1,3-glucanase (BbGlc17A) from Bacteroidetes bacterium from a microbial mat metagenome from the Great Salt Lake was identified. BbGlc17A catalyzed the hydrolytic conversion of laminarin into β-glucooligosaccharides with polymerization degrees of 3–8. The optimal catalytic conditions of BbGlc17A were pH 6.5 and 30 °C. In addition to its hydrolytic activity, BbGlc17A also exhibited transglycosidase activities, involving catalysis of the formation of new β-1,6-glycosidic bonds. BbGlc17A exhibits the classic (β/α)8 TIM-barrel structure and possesses an elongated catalytic groove, distinguishing it from other typical β-1,3-glucanases, which promote the forward direction of the transglycoside reaction. This effectively highlights the potential of the enzyme to convert β-1,3-glucan into mixed functional oligosaccharides. These results reveal the catalytic properties and the application potential of the GH family 17 β-1,3-glucanase and provide valuable information about the group of carbohydrate-active enzymes in biochemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


