黄色发光手性硅纳米粒子的制备以及赖氨酸对映体的荧光/比色双模式识别和纳米生物成像技术
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
长波长发射荧光手性硅纳米粒子(c-SiNPs)因其卓越的光学特性,在生物成像和复杂样品分析方面具有巨大潜力。然而,这些材料的合成仍然是一个相当大的挑战。赖氨酸的活性与其构型有内在联系,因此开发一种快速、灵敏、选择性的方法来区分生化和生物医学领域中的赖氨酸对映体至关重要。本研究创新性地以N-[3-(三甲氧基硅基)丙基]乙二胺和绿原酸为前驱体,通过调整实验参数,首次在室温下合成了发射波长为572 nm的黄色c-SiNPs。所获得的 c-SiNPs 具有优异的光学性能、稳定性和细胞相容性。此外,c-SiNPs 对赖氨酸对映体具有出色的荧光和比色识别能力。这些方法对 d-lysine 的线性范围分别为 0.050-20 mM 和 0.10-30 mM,检测限分别为 7.5 μM 和 17 μM。此外,c-SiNPs 还能对 HeLa 细胞内的 d-lysine 进行生物成像。利用密度泛函理论计算识别机制,并将其与荧光和紫外可见吸收光谱数据相关联,结果证实识别机制与 c-SiNPs 和赖氨酸对映体之间的吉布斯自由能、结合能和氢键数差异有关。本研究开发的 c-SiNPs 制备方法为合成发射波长更长的荧光 c-SiNPs 提供了参考。此外,所建立的赖氨酸对映体鉴别方法对高纯度赖氨酸的使用具有重要的指导意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
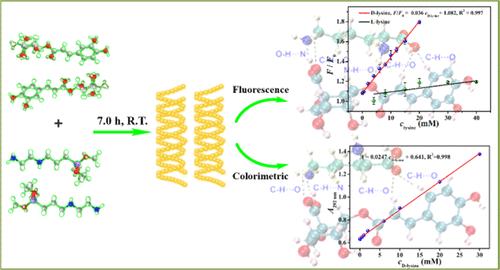
Fabrication of Yellow-Emitting Chiral Silicon Nanoparticles and Fluorescence/Colorimetric Dual-Mode Recognition of Lysine Enantiomers together with Nanobioimaging
Long-wavelength emission fluorescent chiral silicon nanoparticles (c-SiNPs) hold significant potential for biological imaging and complex sample analysis due to their superior optical properties. However, the synthesis of these materials remains a considerable challenge. The activity of lysine is intrinsically linked to its configuration, making it crucial to develop a rapid, sensitive, and selective method for differentiating lysine enantiomers in biochemical and biomedical fields. In this study, N-[3-(trimethoxysilyl)propyl]ethylenediamine and chlorogenic acid were innovatively employed as precursors, and the yellow-emitting c-SiNPs with an emission wavelength of 572 nm were synthesized at room temperature for the first time by adjusting experimental parameters. The obtained c-SiNPs exhibited excellent optical properties, stability, and cell compatibility. Furthermore, the c-SiNPs demonstrated outstanding fluorescence and colorimetric recognition capabilities for lysine enantiomers. Consequently, fluorescence/colorimetric dual-mode sensing methods with high selectivity and sensitivity for the recognition of lysine enantiomers were established, and the linear ranges of these methods for d-lysine were 0.050–20 and 0.10–30 mM, with detection limits of 7.5 and 17 μM, respectively. Additionally, the c-SiNPs demonstrated an ability to bioimaging d-lysine within HeLa cells. Using density functional theory to calculate the recognition mechanism and correlating this with fluorescence and ultraviolet–visible (UV–vis) absorption spectra data, it was confirmed that the recognition mechanism was associated with the Gibbs free energy, binding energy, and hydrogen bond number difference between the c-SiNPs and lysine enantiomers. The method developed in this study for preparing c-SiNPs provided a reference for synthesizing fluorescent c-SiNPs with longer emission wavelengths. Moreover, the established method for identifying lysine enantiomers holds significant guiding implications for the use of high-purity lysine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


