木质素磺酸盐与花青素之间形成的复合物随 pH 值变化的新见解:对颜色和氧化稳定性的影响
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
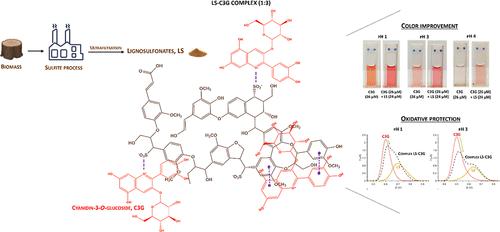
花青素作为天然着色剂和抗氧化剂的应用有限,原因是其在某些条件下会失色且不稳定。本研究采用多种技术方法探讨了木质素磺酸盐(LS)与花青素-3-O-葡萄糖苷(C3G)之间复合物的形成,以及在酸性介质中对 C3G 的红色、氧化稳定性和抗氧化活性的影响。所有数据都表明 LS-C3G 的相互作用与 pH 值有关。热力学参数显示出弱的非共价相互作用,主要是静电作用、氢键和疏水作用,pH 值为 3 时关联常数较高。荧光淬灭和寿命实验分别显示了在 pH 值为 1 和 3 时的静态淬灭和动态淬灭。紫外可见光谱显示,复合物形成时会发生浴色偏移,而在 pH 值为 3 和 4 时,由于 C3G 的红色得到改善,因此会产生高色效应。电化学结果表明,在 pH 值为 3 时,LS 可通过长期保护 C3G 的可氧化分子来增强其稳定性,并提高复合物中花青素的抗氧化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
New Insights into pH-dependent Complex Formation between Lignosulfonates and Anthocyanins: Impact on Color and Oxidative Stability
Anthocyanins have limited application as natural colorants and antioxidants due to their color loss and instability under certain conditions. This research explores the formation of a complex between lignosulfonates (LS) and cyanidin-3-O-glucoside (C3G) using a multitechnique approach as well as the effect on C3G′s red color, oxidative stability, and antioxidant activity in acidic mediums. All data revealed pH-dependent LS-C3G interactions. The thermodynamic parameters showed weak noncovalent interactions, mainly electrostatic interactions, hydrogen bonds, and hydrophobic effect, with a higher association constant determined at pH 3. Fourier-transform infrared spectroscopy and Zeta-potential experiments further corroborate evidence of these LS-C3G interactions. Fluorescence quenching and lifetime experiments revealed static and dynamic quenching at pH 1 and 3, respectively. UV–visible spectroscopy demonstrated a bathochromic shift upon complex formation and a hyperchromic effect at pH 3 and 4, as a consequence of the improved red color of C3G. Electrochemical results suggested that at pH 3 the LS enhances C3G stability by protecting its oxidizable moieties over time, as well as improving the antioxidant activity of the anthocyanin in the complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


