传统发酵大豆凝乳中乌玛米-ACE 抑制肽的鉴定与表征
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
发酵大豆凝乳(FSC)因其鲜美的口感而广受欢迎。其生物活性令人感兴趣。使用纳米高效液相色谱-质谱/质谱鉴定了 FSC 中的肽,并使用各种数据库筛选了 11 种具有潜在鲜味和 ACE 抑制活性的候选肽。药理模型分析表明,这些肽具有很高的 ACE 抑制活性,拟合值为 2,表明这些肽主要通过氢键、静电和疏水相互作用与味觉受体和 ACE 结合。此外,它们的对接和相互作用能与肽的长度无关。合成了三种高鲜味-ACE抑制肽(VE、FEF和WEEF)。它们的鲜味阈值分别为 WEEF (0.32 mM) < FEF (0.55 mM) < VE (1.10 mM),而它们的 IC50 分别为 WEEF (85 ± 2 μM) < FEF (170 ± 10 μM) < VE (205 ± 5 μM)。NO 和 ET-1 的产生与剂量有关,WEEF 显示出最佳的 ACE 抑制活性。研究结果有助于从发酵豆制品中鉴定有效的鲜味剂和 ACE 抑制肽。这也是筛选潜在鲜味剂和 ACE 抑制肽的有用方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
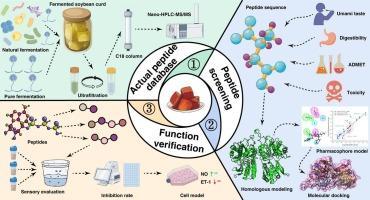
Identification and characterization of umami-ACE inhibitory peptides from traditional fermented soybean curds
Fermented soybean curds (FSC) are popular because of its umami taste. Its bioactivities are of interest. Peptides in FSC were identified using nano-HPLC-MS/MS, and 11 candidate peptides showing potential umami and ACE inhibitory activities were screened using various databases. Pharmacophore model analysis showed their high probability of ACE inhibition with fit values >2, which showed the peptides bound to umami receptors and ACE mainly through hydrogen bond, and electrostatic and hydrophobic interactions. Additionally, their docking and interaction energy were independent of the peptide length. Three high umami-ACE inhibitory peptides (VE, FEF, and WEEF) were synthesized. Their umami thresholds were WEEF (0.32 mM) < FEF (0.55 mM) < VE (1.10 mM), while their IC50 were WEEF (85 ± 2 μM) < FEF (170 ± 10 μM) < VE (205 ± 5 μM). NO and ET-1 production were dose-dependent with WEEF showing the best ACE inhibitory activity. The results allowed identification of effective umami agents and ACE inhibitory peptides from fermented soybean products. It could also be useful method for screening potential umami-ACE inhibitory peptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


