C3N4 中 N 空位和 K 单原子的双位工程:实现光催化的空间电荷传输通道
IF 8.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氮化石墨碳(C3N4)具有合适的带隙和聚合物特性,是一种前景广阔的光催化剂,但其效率却受到光诱导电子/空穴(e-/h+)对分离不良的限制。为解决这一问题,我们建议通过柠檬酸钾和三聚氰胺脲单体的自组装,在层内产生 N 空位,并在 C3N4 层之间桥接 K 单原子。N 空位的引入破坏了 C3N4 的对称性,促进了电子沿分散的 π 共轭网络转移,而 K 原子的存在则通过形成 N-K-N 桥为层间的电子转移提供了通道,从而显著增强了 e-/h+ 对在空间维度上的分离和转移。正如预期的那样,含有 N 空位和 K 单原子的共修饰 C3N4(命名为 CN-K-VN)表现出优异的光催化性能,对四环素的反应速率常数为 9.69×10-2 min-1(在实际水环境中为 7.39×10-2 min-1),在 20 分钟内实现了对四环素 80% 的降解。该研究对反应机理以及降解中间产物的毒性进行了深入探讨。这项研究提供了一种增强光催化剂电子空间分离的策略,凸显了光催化剂在光催化中的重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
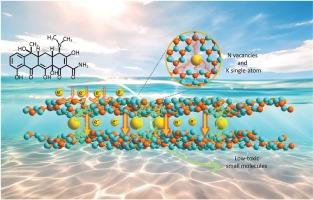

Dual-site engineering of N vacancies and K single-atoms in C3N4: Enabling spatial charge transfer channels for photocatalysis
Graphitic carbon nitride (C3N4) is a promising photocatalyst due to its suitable band gap and polymer properties, but its efficiency is limited by the poor separation of photoinduced electron/hole (e–/h+) pairs. To address this issue, we propose creating N vacancies within the layers and bridging K single-atoms between the C3N4 layers through the self-assembly of potassium citrate and melamine–urea monomers. The introduction of N vacancies disrupts the symmetry of C3N4, promoting electron transfer along the delocalized π-conjugated network, while the presence of K atoms provides channels for electron transfer between the layers by forming N![]() K
K![]() N bridges, thereby leading to significant enhancement in the separation and transfer of e–/h+ pairs across spatial dimension. As expected, the co-modified C3N4, with N vacancies and K single-atoms (designated as CN-K-VN), exhibits excellent photocatalytic performance, with reaction rate constant of 9.69 × 10−2 min−1 (7.39 × 10−2 min−1 in real water environment) for tetracycline, achieving 80% degradation of tetracycline within 20 min. The reaction mechanism, as well as the toxicity of the degradation intermediates, is deeply discussed. This study provides a strategy to enhance the spatial separation of electrons for photocatalyst, highlighting its significance role in photocatalysis.
N bridges, thereby leading to significant enhancement in the separation and transfer of e–/h+ pairs across spatial dimension. As expected, the co-modified C3N4, with N vacancies and K single-atoms (designated as CN-K-VN), exhibits excellent photocatalytic performance, with reaction rate constant of 9.69 × 10−2 min−1 (7.39 × 10−2 min−1 in real water environment) for tetracycline, achieving 80% degradation of tetracycline within 20 min. The reaction mechanism, as well as the toxicity of the degradation intermediates, is deeply discussed. This study provides a strategy to enhance the spatial separation of electrons for photocatalyst, highlighting its significance role in photocatalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materiomics
Materials Science-Metals and Alloys
CiteScore
14.30
自引率
6.40%
发文量
331
审稿时长
37 days
期刊介绍:
The Journal of Materiomics is a peer-reviewed open-access journal that aims to serve as a forum for the continuous dissemination of research within the field of materials science. It particularly emphasizes systematic studies on the relationships between composition, processing, structure, property, and performance of advanced materials. The journal is supported by the Chinese Ceramic Society and is indexed in SCIE and Scopus. It is commonly referred to as J Materiomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


