经磷脂酶水解的液态蛋黄的特性:结构、热稳定性和乳化特性
IF 7
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
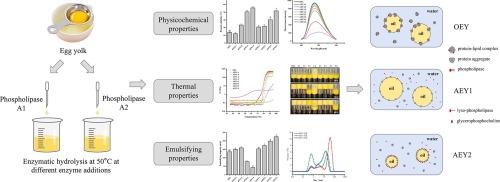
本研究旨在阐明磷脂酶 A1(PLA1)和磷脂酶 A2(PLA2)在水解蛋黄(EY)方面的差异。结果表明,磷脂酶水解后,脂蛋白胶束结构解体,从而提高了蛋白质的溶解度。经 PLA1 和 PLA2 处理后的溶解度(91.36%/83.49%)明显高于未处理蛋黄的溶解度(27.89%)。与此同时,脂蛋白胶束结构的解体导致蛋白质结构的展开,疏水链被埋在空间结构内部,而带电氨基酸和亲水链则暴露在表面。这种结构变形有助于提高 EY 的热稳定性,从而增加分子间的静电排斥。相比之下,水解 EY 的 PLA1 的热稳定性要比 PLA2 好,这是因为 PLA1 的表面疏水性较低。然而,由于生成的 1-异磷脂酶具有更好的稳定性和乳化能力,因此 PLA2 水解 EY(最多 225 mL)的乳化能力大大高于 PLA1(最多 159 mL)。此外,我们还发现蛋白质和磷脂在界面上共同发挥作用,影响乳化液的粒度和稳定性。具体来说,磷脂的乳化活性在决定粒度方面可能起着决定性作用,而蛋白质或蛋白质颗粒的界面吸附在确保乳液稳定性方面可能更为关键。这些发现对磷脂酶催化蛋黄水解的应用和发展具有重要意义,为生产具有高热稳定性或高乳化能力的 EY 提供了实际指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization of liquid egg yolks hydrolyzed by phospholipase: Structure, thermal stability and emulsification properties
This study aims to clarify the difference between phospholipase A1 (PLA1) and phospholipase A2 (PLA2) in terms of hydrolyzing egg yolk (EY). The results indicated that the disintegration of the lipoprotein micelle structure after phospholipase hydrolysis induced an enhanced solubility of proteins. The solubility after PLA1 and PLA2 treatment (91.36 %/83.49 %) was significantly higher than that of the untreated egg yolk (27.89 %). Simultaneously, the disintegration of the lipoprotein micelle structure induced structural unfolding of proteins with hydrophobic chains buried inside the spatial structure, while charged amino acids and hydrophilic chains exposed on the surface. This structural deformation contributed to the increased thermal stability of EY, thereby increasing intermolecular electrostatic repulsion. In comparison, PLA1 hydrolyzed EY showed relatively better thermal stability than PLA2, due to the lower surface hydrophobicity. However, PLA2 hydrolyzed EY (up to 225 mL) had greatly higher emulsifying capacity than PLA1 (up to 159 mL), due to the better stability and emulsifying ability of the generated 1-lyso-phospholipase. Furthermore, we discovered that proteins and phospholipids jointly functioned at the interface to influence the particle size and stability of emulsions. Specifically, the emulsifying activity of phospholipids may play a more decisive role in determining the particle size, while the interfacial adsorption of proteins or protein particles may be more crucial in ensuring the stability of the emulsions. These findings had significant implications for the application and advancement of phospholipase-catalyzed egg yolk hydrolysis, providing practical guidance for the production of EY with high thermal stability or emulsifying capacity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


