揭示氯化铝酞菁对大鼠肝线粒体生物能和氧化状态的内在和光动力效应。
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
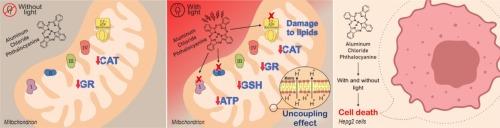
以往的研究表明,线粒体是光动力疗法(PDT)的一个重要靶点,可用作包括肝细胞癌(HCC)在内的多种恶性肿瘤的治疗方法。在这项研究中,我们研究了光敏剂(PS)氯化铝酞菁(AlClPc)对线粒体功能的内在毒性和光动力效应。通过差速离心法从大鼠肝脏中分离出线粒体,对线粒体的生物能、结构和氧化状态等几个方面进行了研究。此外,实验还证明了 AlClPc 对 HepG2 细胞活力的内在和光动力效应。无论光刺激与否,AlClPc 都能与线粒体相互作用;然而,在最大使用浓度(40 μM)下,光刺激会降低其与线粒体的相互作用。虽然 AlClPc 本身会阻碍过氧化氢酶(CAT)和谷胱甘肽还原酶(GR)的活性,但在没有光刺激的情况下,它没有明显的能力产生氧化应激或影响线粒体的生物能,这也是人们对理想 PS 的预期。然而,在光照下,AlClPc 会对线粒体功能产生严重的不利影响,加强其对 CAT 的内在抑制作用,产生氧化应激,破坏线粒体的生物能。在氧化应激参数方面,AlClPc 会诱发脂质过氧化反应,并降低线粒体中还原型谷胱甘肽(GSH)的水平。在生物能方面,AlClPc 促进氧化磷酸化解偶联和复合体 I、复合体 II 和 FoF1-ATP 合酶复合体的光动力失活,从而降低线粒体 ATP 产量。最后,无论是否存在光刺激,AlClPc 都会使 HepG2 细胞的存活率出现浓度依赖性下降。虽然 AlClPc 对线粒体生物能的有害光动力效应为治疗 HCC 和其他恶性肿瘤带来了希望,但其对 HepG2 细胞固有的毒性影响强调了谨慎应用的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unraveling the intrinsic and photodynamic effects of aluminum chloride phthalocyanine on bioenergetics and oxidative state in rat liver mitochondria
Previous research has revealed that mitochondria are an important target for photodynamic therapy (PDT), which might be employed as a therapeutic approach for several malignancies, including hepatocellular carcinoma (HCC). In this study, we investigated both intrinsic toxicity and photodynamic effects of the photosensitizer (PS) aluminum chloride phthalocyanine (AlClPc) on mitochondrial functions. Several aspects of mitochondrial bioenergetics, structure, and oxidative state were investigated in the isolated mitochondria obtained from rat liver by differential centrifugation. Additionally, experiments were conducted to demonstrate the intrinsic and photodynamic effects of AlClPc on the viability of HepG2 cells. AlClPc interacted with mitochondria regardless of photostimulation; however, at the maximum utilized concentration (40 μM), photostimulation reduced its interaction with mitochondria. Although AlClPc hindered catalase (CAT) and glutathione reductase (GR) activities intrinsically, it had no discernable capacity to generate oxidative stress or impact bioenergetics in mitochondria without photostimulation, as one would anticipate from an ideal PS. When exposed to light, however, AlClPc had a substantially unfavorable influence on mitochondrial function, strengthening its intrinsic inhibitory action on CAT, producing oxidative stress, and jeopardizing mitochondrial bioenergetics. In terms of oxidative stress parameters, AlClPc induced lipid peroxidation and decreased the level of reduced glutathione (GSH) in mitochondria. Regarding bioenergetics, AlClPc promoted oxidative phosphorylation uncoupling and photodynamic inactivation of complex I, complex II, and the FoF1–ATP synthase complex, lowering mitochondrial ATP production. Lastly, AlClPc exhibited a concentration-dependent decrease in the viability of HepG2 cells, regardless of the presence or absence of photostimulation. While the harmful photodynamic effects of AlClPc on mitochondrial bioenergetics hold promise for treating HCC and other malignancies, the inherent toxic impacts on HepG2 cells underscore the need for caution in its application for this purpose.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


