苯甲酸能增强肉鸡肠道 IgA 对秋伤寒沙门氏菌感染的反应。
IF 4.2
1区 农林科学
Q1 AGRICULTURE, DAIRY & ANIMAL SCIENCE
引用次数: 0
摘要
作为一种饲料添加剂,苯甲酸(BA)已被证明可显著提高饲料转化效率、调节胃肠道 pH 值并改善动物整体健康。幼畜极易感染鼠伤寒杆菌,死亡率高,经济损失巨大。尽管有迹象表明 BA 在提高肠道免疫力方面具有良好的免疫调节潜力,但对其潜在机制的了解仍然不够。本研究探讨了 BA 如何通过免疫调节途径增强幼年动物的肠道抗感染防御能力,重点关注其对巨噬细胞极化和 IgA 介导的免疫反应的影响。通过体外细胞实验和动物模型,我们研究了BA治疗后巨噬细胞表型的改变。我们通过免疫荧光染色、Western 印迹和实时定量 PCR 评估了肠道中免疫相关基因的表达。结果表明,BA 通过激活 mTOR/PPAR-γ/STAT3 信号通路促进 M2 巨噬细胞极化。此外,BA 还能增强肠道中聚合免疫球蛋白受体(PIgR)、TNF 家族的 B 细胞活化因子(BAFF)和活化诱导胞苷脱氨酶(AID)的表达,从而提高 B 细胞的 IgA 产量。这些结果凸显了 BA 在增强幼鸡先天性免疫功能、减轻伤寒杆菌感染造成的肠道损伤以及最终促进动物健康和食品安全方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
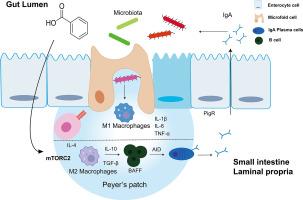
Benzoic Acid potentiates intestinal IgA response in broiler chickens against Salmonella enterica Serovar Typhimurium infection
As a feed additive, Benzoic Acid (BA) has been demonstrated to significantly enhance feed conversion efficiency, regulate gastrointestinal pH, and improve overall animal health. Young animals, highly susceptible to S. Typhimurium infection, suffer from high mortality rates and substantial economic losses due to this pathogen. Despite promising indications of BA's immunomodulatory potential in boosting intestinal immunity, its underlying mechanisms remain insufficiently understood. This study investigates how BA strengthens intestinal anti-infection defenses in young animals via immunomodulatory pathways, focusing on its impact on macrophage polarization and IgA-mediated immune responses. Employing in vitro cell experiments and animal models, we examined the macrophage phenotypic alterations following BA treatment. We assessed the expression of immune-related genes in the intestine through immunofluorescence staining, Western blotting, and quantitative real-time PCR. The results demonstrate that BA promotes M2 macrophage polarization by activating the mTOR/PPAR-γ/STAT3 signaling pathways. Furthermore, BA enhances the intestinal expression of the polymeric immunoglobulin receptor (PIgR), B-cell activating factor (BAFF) from the TNF family, and activation-induced cytidine deaminase (AID), thereby enhancing IgA production by B-cells. These results underscore the potential of BA to bolster innate immune functions in young chickens, mitigate intestinal damage caused by S. Typhimurium infection, and ultimately promote both animal health and food safety.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Poultry Science
农林科学-奶制品与动物科学
CiteScore
7.60
自引率
15.90%
发文量
0
审稿时长
94 days
期刊介绍:
First self-published in 1921, Poultry Science is an internationally renowned monthly journal, known as the authoritative source for a broad range of poultry information and high-caliber research. The journal plays a pivotal role in the dissemination of preeminent poultry-related knowledge across all disciplines. As of January 2020, Poultry Science will become an Open Access journal with no subscription charges, meaning authors who publish here can make their research immediately, permanently, and freely accessible worldwide while retaining copyright to their work. Papers submitted for publication after October 1, 2019 will be published as Open Access papers.
An international journal, Poultry Science publishes original papers, research notes, symposium papers, and reviews of basic science as applied to poultry. This authoritative source of poultry information is consistently ranked by ISI Impact Factor as one of the top 10 agriculture, dairy and animal science journals to deliver high-caliber research. Currently it is the highest-ranked (by Impact Factor and Eigenfactor) journal dedicated to publishing poultry research. Subject areas include breeding, genetics, education, production, management, environment, health, behavior, welfare, immunology, molecular biology, metabolism, nutrition, physiology, reproduction, processing, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


