补充乳酸菌可改善 iNOS 基因敲除小鼠的代谢稳定性。
IF 3.4
3区 医学
Q2 NUTRITION & DIETETICS
引用次数: 0
摘要
氧化应激和亚硝基应激在正常生理过程和代谢紊乱的发病机制中起着关键作用。我们实验室以前的研究表明,iNOS-/-小鼠存在胰岛素抵抗(IR)和血脂异常,这强调了维持最佳氧化还原平衡的重要性。这些小鼠的肠道微生物群发生了改变,乳酸杆菌减少。因此,我们假设补充乳酸菌可以缓解 iNOS-/- 小鼠的代谢紊乱。为了验证这一假设,我们将 iNOS-/- 小鼠和野生型(WT)小鼠分为四组:补充或不补充乳酸杆菌的 iNOS-/- 小鼠组、补充或不补充乳酸杆菌的 WT 小鼠组,并监测糖耐量、胰岛素抵抗、糖代谢、血脂、与糖和脂代谢相关的基因表达(qPCR)、粪便肠道微生物群(16S rRNA 测序)以及血清和盲肠代谢组学(LC-MS)。红外和血脂异常 iNOS-/- 小鼠表现出微生物多样性降低、乳酸杆菌减少和血清代谢物改变,表明代谢失调。与 WT 小鼠相比,在 iNOS-/- 小鼠体内补充乳酸菌可有效逆转葡萄糖耐受不良、红外热、血脂异常以及相关的代谢异常。与未经治疗的 iNOS-/- 小鼠相比,这些改善与肝脏和脂肪组织中脂肪酸合成、肝脏中脂质氧化和肠道组织中脂质外流相关基因表达的变化有关。尽管乳酸菌对代谢指标有积极影响,但补充乳酸菌并不能减轻体重或纠正能量平衡失调,这体现在 iNOS-/- 小鼠的 VCO2 产生量、发热量和代谢率降低。研究结果表明,补充乳酸菌可改善代谢紊乱,但并不能完全恢复被破坏的能量平衡,这凸显了肠道微生物组与代谢之间复杂的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
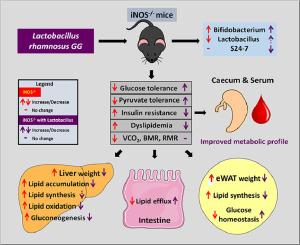
Improved metabolic stability in iNOS knockout mice with Lactobacillus supplementation
Oxidative and nitrosative stress play pivotal roles in normal physiological processes and the pathogenesis of metabolic disorders. Previous studies from our lab demonstrated insulin resistance (IR), and dyslipidemia in iNOS−/− mice, emphasizing the importance of maintaining optimal redox balance. These mice exhibited altered gut microbiota with decreased Lactobacillus. Therefore, we hypothesized that Lactobacillus supplementation could mitigate metabolic disturbances in iNOS−/− mice. To test this hypothesis, iNOS−/− mice and wild-type (WT) mice were divided into four groups: iNOS−/- with or without Lactobacillus supplementation, WT with or without Lactobacillus supplementation and glucose tolerance, insulin resistance, gluconeogenesis, lipids, gene expression related to glucose and lipid metabolism (qPCR), fecal gut microbiota (16S rRNA sequencing), and serum and caecum metabolomics (LC-MS) were monitored. IR and dyslipidemic iNOS−/− mice exhibited reduced microbial diversity, diminished presence of Lactobacillus, and altered serum metabolites, indicating metabolic dysregulation. Lactobacillus supplementation in iNOS−/− mice effectively reversed glucose intolerance, IR, dyslipidemia, and associated metabolic irregularities compared to WT. These improvements correlated with changes in gene expression related to fatty acid synthesis in liver and adipose tissue, lipid oxidation in liver, and lipid efflux in intestinal tissue as compared to untreated iNOS−/− mice. Despite the positive effects on metabolic markers, Lactobacillus supplementation did not reduce body weight or rectify disrupted energy balance, as evidenced by reduced VCO2 production, heat generation, and metabolic rates in iNOS−/− mice. The results suggest that Lactobacillus supplementation ameliorates metabolic disturbances but did not fully restore disrupted energy balance, highlighting complex interactions between the gut microbiome and metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nutrition Research
医学-营养学
CiteScore
7.60
自引率
2.20%
发文量
107
审稿时长
58 days
期刊介绍:
Nutrition Research publishes original research articles, communications, and reviews on basic and applied nutrition. The mission of Nutrition Research is to serve as the journal for global communication of nutrition and life sciences research on diet and health. The field of nutrition sciences includes, but is not limited to, the study of nutrients during growth, reproduction, aging, health, and disease.
Articles covering basic and applied research on all aspects of nutrition sciences are encouraged, including: nutritional biochemistry and metabolism; metabolomics, nutrient gene interactions; nutrient requirements for health; nutrition and disease; digestion and absorption; nutritional anthropology; epidemiology; the influence of socioeconomic and cultural factors on nutrition of the individual and the community; the impact of nutrient intake on disease response and behavior; the consequences of nutritional deficiency on growth and development, endocrine and nervous systems, and immunity; nutrition and gut microbiota; food intolerance and allergy; nutrient drug interactions; nutrition and aging; nutrition and cancer; obesity; diabetes; and intervention programs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


