拟南芥蛋白质磷酸化、乙酰化和 β-羟基丁酰化的全球剖析
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
蛋白质翻译后修饰(PTMs)调控蛋白质功能,但在拟南芥中仍鲜为人知。本研究考察了三种 PTM:赖氨酸乙酰化(Kac)、赖氨酸β-羟基丁酰化(Kbhb)和磷酸化。通过 LC-MS/MS,我们在 2455、3109 和 2786 个蛋白质中分别发现了 4571 个 Kac 位点、7812 个 Kbhb 位点和 6237 个磷酸化位点。亚细胞定位分析表明,Kac 和 Kbhb 蛋白之间有明显的重叠,主要分布在叶绿体、细胞质和细胞核中,而磷酸化蛋白则主要分布在细胞核和叶绿体中。基团分析强调了修饰位点周围特定氨基酸的富集,其中有几个基团是保守的。此外,有 529 个蛋白质同时含有这三种 PTM,突显了潜在的调控相互作用。Kac、Kbhb和磷酸化蛋白在糖酵解、TCA循环、碳固定和脂质代谢途径中特别丰富,影响着能量的产生和脂质的积累。根据以往营养限制条件下的转录组数据,这些频繁修饰的关键酶似乎是非生物胁迫响应的重要组成部分。与 Kac 和 Kbhb 相关的组蛋白修饰的存在也可能表明了基因表达和胁迫适应的表观遗传调控。N. oceanica 全面的 PTM 图谱为未来的代谢工程和生物技术应用提供了宝贵的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
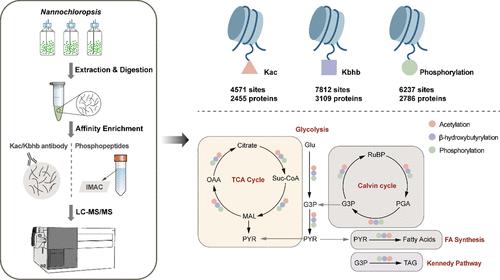
Global Profiling of Protein Phosphorylation, Acetylation, and β-Hydroxybutyrylation in Nannochloropsis oceanica
Protein post-translational modifications (PTMs) regulate protein functions but remain poorly characterized in Nannochloropsis. This study examined three PTMs: lysine acetylation (Kac), lysine β-hydroxybutyrylation (Kbhb), and phosphorylation. Using LC-MS/MS, we identified 4571 Kac sites, 7812 Kbhb sites, and 6237 phosphorylation sites across 2455, 3109, and 2786 proteins, respectively. Subcellular localization analysis revealed significant overlaps between Kac and Kbhb proteins, primarily in the chloroplast, cytosol, and nucleus, while phosphorylated proteins were predominantly located in the nucleus and chloroplast. Motif analysis highlighted specific amino acid enrichments around modification sites, with several motifs conserved. Additionally, 529 proteins harbored all three PTMs, underscoring the potential regulatory interplay. Kac, Kbhb, and phosphorylated proteins were particularly abundant in glycolysis, the TCA cycle, carbon fixation, and lipid metabolism pathways, influencing energy production and lipid accumulation. Based on previous transcriptome data under nutrient-limited conditions, these frequently modified key enzymes appear to be vital components in the response to abiotic stress. The presence of histone modifications related to Kac and Kbhb might also point to the epigenetic regulation in gene expression and stress adaptation. This comprehensive PTM landscape in N. oceanica provides a foundation of valuable insights into future metabolic engineering and biotechnological applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


