调节纤维化机械微环境以治疗特发性肺纤维化
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
特发性肺纤维化(IPF)会因损伤性机械力而恶化,这些机械力会破坏肺部机械微环境的平衡,导致肺泡功能障碍并加重疾病的严重程度。然而,鉴于纤维化肺固有的机械敏感性,其中 II 型肺泡上皮细胞(AEC II)受到持续的拉伸,过度激活的肌成纤维细胞在机械传导过程中经历恶性相互作用,因此开发有效的策略来调节肺机械微环境变得势在必行。在此,研究人员构建了环(RGDfC)肽装饰的沸石咪唑酸框架-8纳米粒子(命名为ZDFPR NPs),以靶向修复病变肺部异常的机械力水平。具体而言,通过 pH 响应型 ZDFPR NPs 释放锌离子和 7,8-二羟基黄酮,降低 AEC II 的机械张力,促进肺泡修复和分化。同时,在机械传导过程中,肌成纤维细胞收缩性与细胞外基质硬度之间的恶性相互作用会被法舒地尔抑制ROCK信号通路所破坏。结果表明,ZDFPR NPs成功恢复了博莱霉素诱导的纤维化小鼠的肺机械稳态,并终止了其纤维化进程。这项研究不仅为调节肺机械微环境提供了一种前景广阔的策略,还为治疗 IPF 开辟了一条新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
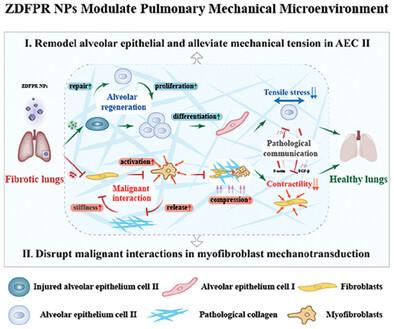
Modulating Fibrotic Mechanical Microenvironment for Idiopathic Pulmonary Fibrosis Therapy
Idiopathic pulmonary fibrosis (IPF) is exacerbated by injurious mechanical forces that destabilize the pulmonary mechanical microenvironment homeostasis, leading to alveolar dysfunction and exacerbating disease severity. However, given the inherent mechanosensitivity of fibrotic lungs, where type II alveolar epithelial cells (AEC IIs) are subjected to persistent stretching and overactivated myofibroblasts experience malignant interactions during mechanotransduction, it becomes imperative to develop effective strategies to modulate the pulmonary mechanical microenvironment. Herein, cyclo (RGDfC) peptide-decorated zeolitic imidazolate framework-8 nanoparticles (named ZDFPR NPs) are constructed to target and repair the aberrant mechanical force levels in pathological lungs. Specifically, reduces mechanical tension in AEC IIs by pH-responsive ZDFPR NPs that release zinc ions and 7, 8-dihydroxyflavone to promote alveolar repair and differentiation. Meanwhile, malignant interactions between myofibroblast contractility and extracellular matrix stiffness during mechanotransduction are disrupted by the fasudil inhibition ROCK signaling pathway. The results show that ZDFPR NPs successfully restored pulmonary mechanical homeostasis and terminated the fibrosis process in bleomycin-induced fibrotic mice. This study not only presents a promising strategy for modulating pulmonary mechanical microenvironment but also pioneers a novel avenue for IPF treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


