反布雷特烯烃合成问题的解决方案
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
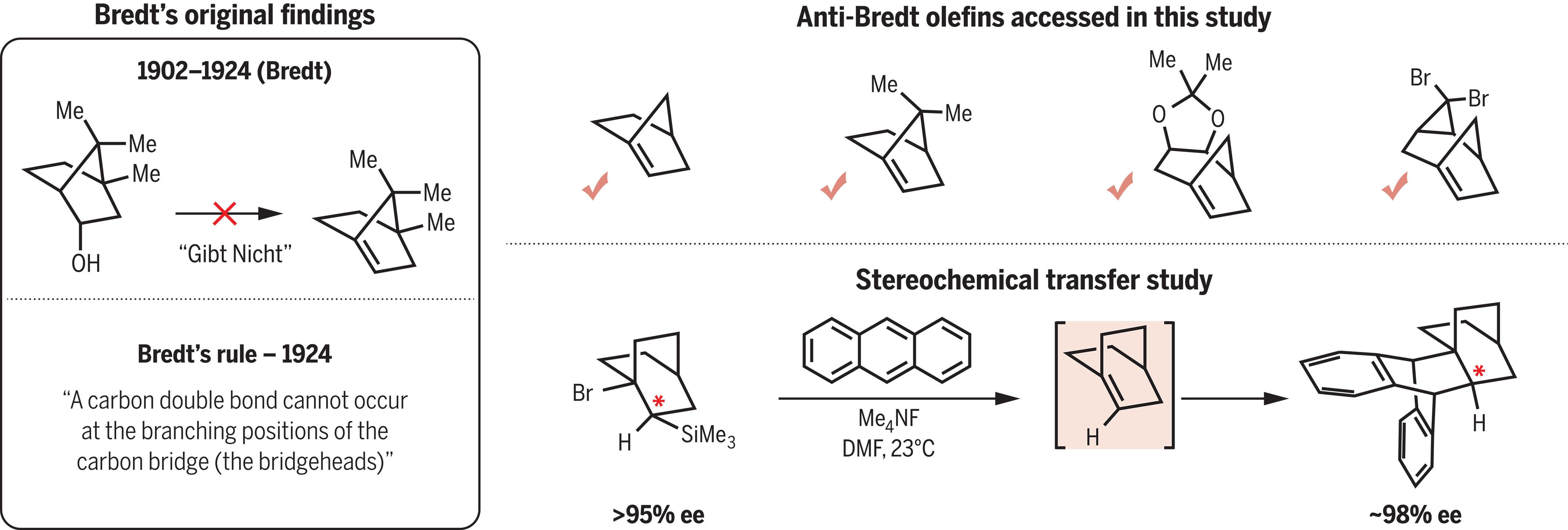
不饱和有机分子中的π键通常具有明确的几何形状,在不同的结构环境中保持不变。然而,这些几何形状可能会发生扭曲,从而导致 π 键的反应性增强。虽然具有弯曲几何结构的含π键化合物在合成化学中得到了很好的利用,但相应地利用具有扭曲或金字塔化结构的含π键化合物的技术仍不发达。我们报告的研究可能是最臭名昭著的一类含有 π 键的几何扭曲分子:反布雷特烯烃 (ABO)。自 1924 年以来,ABOs 就已为人所知,但传统观点认为 ABOs 很难或根本不可能获得。我们为这一长期存在的问题提供了解决方案。我们的研究还突出了对化合物的策略性操作,这些化合物因存在几何约束的 π 键而显示出相当大的畸变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A solution to the anti-Bredt olefin synthesis problem
The π-bonds in unsaturated organic molecules are typically associated with having well-defined geometries that are conserved across diverse structural contexts. Nonetheless, these geometries can be distorted, leading to heightened reactivity of the π-bond. Although π-bond–containing compounds with bent geometries are well utilized in synthetic chemistry, the corresponding leveraging of π-bond–containing compounds that display twisting or pyramidalization remains underdeveloped. We report a study of perhaps the most notorious class of geometrically distorted molecules that contain π-bonds: anti-Bredt olefins (ABOs). ABOs have been known since 1924, and conventional wisdom maintains that ABOs are difficult or impossible to access. We provide a solution to this long-standing problem. Our study also highlights the strategic manipulation of compounds that display considerable distortion arising from the presence of geometrically constrained π-bonds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


