体外药理学分析有助于评估化学品的全身毒性。
IF 3.3
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
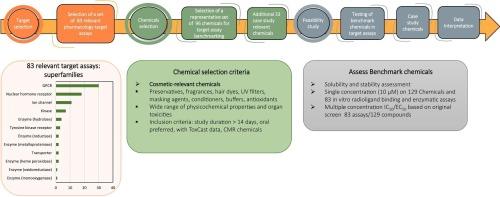
我们开发了一个经过调整的体外药理学分析小组(APPP),其中包括制药业使用的靶标,以及细胞功能与全身毒性相关的其他靶标。这个由 83 个靶标测定组成的小组用于分析 129 种化妆品相关化学品的活性,这些化学品具有不同的化学结构、理化性质和化妆品用途类型。单浓度和浓度-反应数据之间的重现性证明了内部数据的一致性是可靠的,并且与 ToxCast 和药用辅料数据集中报告的数据显示出良好的一致性。我们讨论了如何分析数据,并通过案例研究说明了多种潜在的使用环境,以及其他新方法,以支持不需要新动物研究数据的化妆品化学品风险评估。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In vitro pharmacologic profiling aids systemic toxicity assessment of chemicals
An adapted in vitro pharmacology profiling panel (APPP) was developed that included targets used in the pharmaceutical industry alongside additional targets whose cellular functions have been linked to systemic toxicities. This panel of 83 target assays was used to profile the activities of 129 cosmetic relevant chemicals with diverse chemical structures, physiochemical properties and cosmetic use types. Internal data consistency was proved robust, as evidenced by the reproducibility between single concentration and concentration-response data and showed good concordance with data reported in the ToxCast and drug excipient datasets. We discuss how the data can be analyzed and multiple potential contexts of use illustrated by case studies, alongside other new approach methodologies, to support cosmetic chemical risk assessments that do not require data from new animal studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


