对羟基苯甲酸酯抑制人类和大鼠性腺 3β- 羟基类固醇脱氢酶的结构决定因素。
IF 3.3
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究探讨了 10 种对羟基苯甲酸酯对人类 KGN 细胞和大鼠睾丸微粒体中人类和大鼠性腺 3β- 羟基类固醇脱氢酶(3β-HSD)活性的影响,以及对 KGN 细胞分泌孕酮的影响,并比较了人类和大鼠的抑制效力。结果显示,乙基、丙基、丁基、己基、庚基、壬基、苯基和苄基对羟基苯甲酸酯对人类 3β-HSD2 的 IC50 值从 4.15 μM 到 139.96 μM 不等,显示出混合抑制剂的特征。值得注意的是,在 KGN 细胞中,除壬基对羟基苯甲酸酯和苯基对羟基苯甲酸酯外,所有检测的对羟基苯甲酸酯在 5-50 μM 的浓度下都能显著抑制黄体酮的分泌。在大鼠体内,这些对羟基苯甲酸酯类对性腺 3β-HSD1 的 IC50 值在 7.15 至 110.76 μM 之间波动,同样起到混合抑制剂的作用。通过对接分析,大多数对羟基苯甲酸酯类化合物都能有效地与 NAD+ 和/或类固醇结合位点结合。此外,双变量相关分析揭示了 IC50 值与对羟基苯甲酸酯的结构特征(如 LogP、分子量、重原子数和醇分子中的碳数)之间的反相关性。三维-QSAR 阐明了关键区域,包括氢键供体、氢键受体和疏水区域,其中抑制作用最强的对羟基苯甲酸壬酯与所有区域都有相互作用,而抑制作用最弱的对羟基苯甲酸乙酯则与除疏水区域外的所有区域都有相互作用。总之,这项研究强调了几种对羟基苯甲酸酯对人类和大鼠性腺 3β-HSD 活性的抑制作用,其抑制效力受到疏水性和碳链长度的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
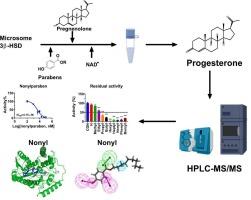
Structural determinants of parabens in inhibiting human and rat gonadal 3β-hydroxysteroid dehydrogenase
This study delved into the impacts of 10 parabens on the activity of human and rat gonadal 3β-hydroxysteroid dehydrogenase (3β-HSD) within human KGN cell and rat testicular microsomes, as well as on the secretion of progesterone in KGN cells and the inhibitory potency was compared between human and rats. Intriguingly, the outcomes revealed that ethyl, propyl, butyl, hexyl, heptyl, nonyl, phenyl, and benzyl parabens displayed varying IC50 values for human 3β-HSD2, from 4.15 to 139.96 μM, demonstrating characteristics of mixed inhibitors. Notably, within KGN cells, all examined parabens, excluding nonyl and phenyl parabens, significantly inhibited progesterone secretion at 5–50 μM. In the case of rats, the IC50 values for these parabens on gonadal 3β-HSD1 fluctuated between 7.15 and 110.76 μM, likewise functioning as mixed inhibitors. Through docking analysis, it was proposed that most parabens effectively bind to NAD+ and/or steroid binding site. Moreover, bivariate correlation analysis unveiled an inverse correlation between IC50 values and structural characteristics such as LogP, molecular weight, heavy atom number, and carbon number within the alcohol moiety of parabens. 3D-QSAR elucidated pivotal regions, comprising hydrogen bond donor, hydrogen bond acceptor, and hydrophobic region, with the most potent inhibitor nonyl paraben engaging with all regions, while the weakest inhibitor ethyl paraben interacted with the regions except for the hydrophobic region. In conclusion, this investigation underscored the inhibitory effects imparted by several parabens on both human and rat gonadal 3β-HSD activity, with their inhibitory potency being modulated by aspects of hydrophobicity and carbon chain length.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


