基于收缩通道的长期稳定多特征微流体阻抗流式细胞仪用于单细胞机械表型分析
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
使用收缩微通道的微流体阻抗流式细胞仪(m-IFC)是高通量测量单细胞机械特性的理想选择。然而,小于细胞的通道容易发生不可逆的堵塞,极大地影响了系统的稳定性和通量。同时,提取单个定量指标(即细胞通过收缩部分的总时间)的常见做法不足以解读单个细胞复杂的机械特性。在此,本研究提出了一种基于收缩通道的长期稳定、多特征 m-IFC,用于单细胞机械表型分析。通过添加微小的黄原胶(XG)聚合物,可以有效克服堵塞问题。细胞能以 500 μL/h 的流速通过收缩通道而不会堵塞,具有高通量(每秒 ∼ 240 个样本)和长期稳定性(∼ 2 小时)。此外,还实现了六个检测区域,以捕捉单细胞通过长收缩通道全过程的多个相关特征,如蠕动、摩擦和松弛阶段。为了验证多特征 m-IFC 的性能,分别检测了细胞骨架内微管和微丝受到扰动的细胞。这表明提取的特征为单细胞结构和机械转变分析提供了更全面的线索。总之,我们提出的多特征 m-IFC 具有无堵塞和高通量的优点,可以推广到其他细胞类型,用于无损和实时的机械表型分析,具有很高的成本效益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
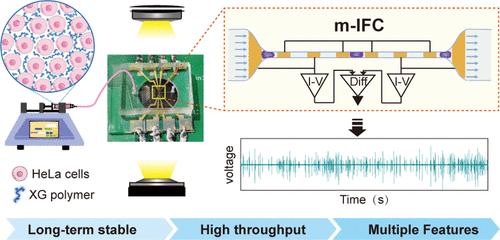
Long-Term Stable and Multifeature Microfluidic Impedance Flow Cytometry Based on a Constricted Channel for Single-Cell Mechanical Phenotyping
The microfluidic impedance flow cytometer (m-IFC) using constricted microchannels is an appealing choice for the high-throughput measurement of single-cell mechanical properties. However, channels smaller than the cells are susceptible to irreversible blockage, extremely affecting the stability of the system and the throughput. Meanwhile, the common practice of extracting a single quantitative index, i.e., total cell passage time, through the constricted part is inadequate to decipher the complex mechanical properties of individual cells. Herein, this study presents a long-term stable and multifeature m-IFC based on a constricted channel for single-cell mechanical phenotyping. The blockage problem is effectively overcome by adding tiny xanthan gum (XG) polymers. The cells can pass through the constricted channel at a flow rate of 500 μL/h without clogging, exhibiting high throughput (∼240 samples per second) and long-term stability (∼2 h). Moreover, six detection regions were implemented to capture the multiple features related to the whole process of a single cell passing through the long-constricted channel, e.g., creep, friction, and relaxation stages. To verify the performance of the multifeature m-IFC, cells treated with perturbations of microtubules and microfilaments within the cytoskeleton were detected, respectively. It suggests that the extracted features provide more comprehensive clues for single-cell analysis in structural and mechanical transformation. Overall, our proposed multifeature m-IFC exhibits the advantages of nonclogging and high throughput, which can be extended to other cell types for nondestructive and real-time mechanical phenotyping in cost-effective applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


