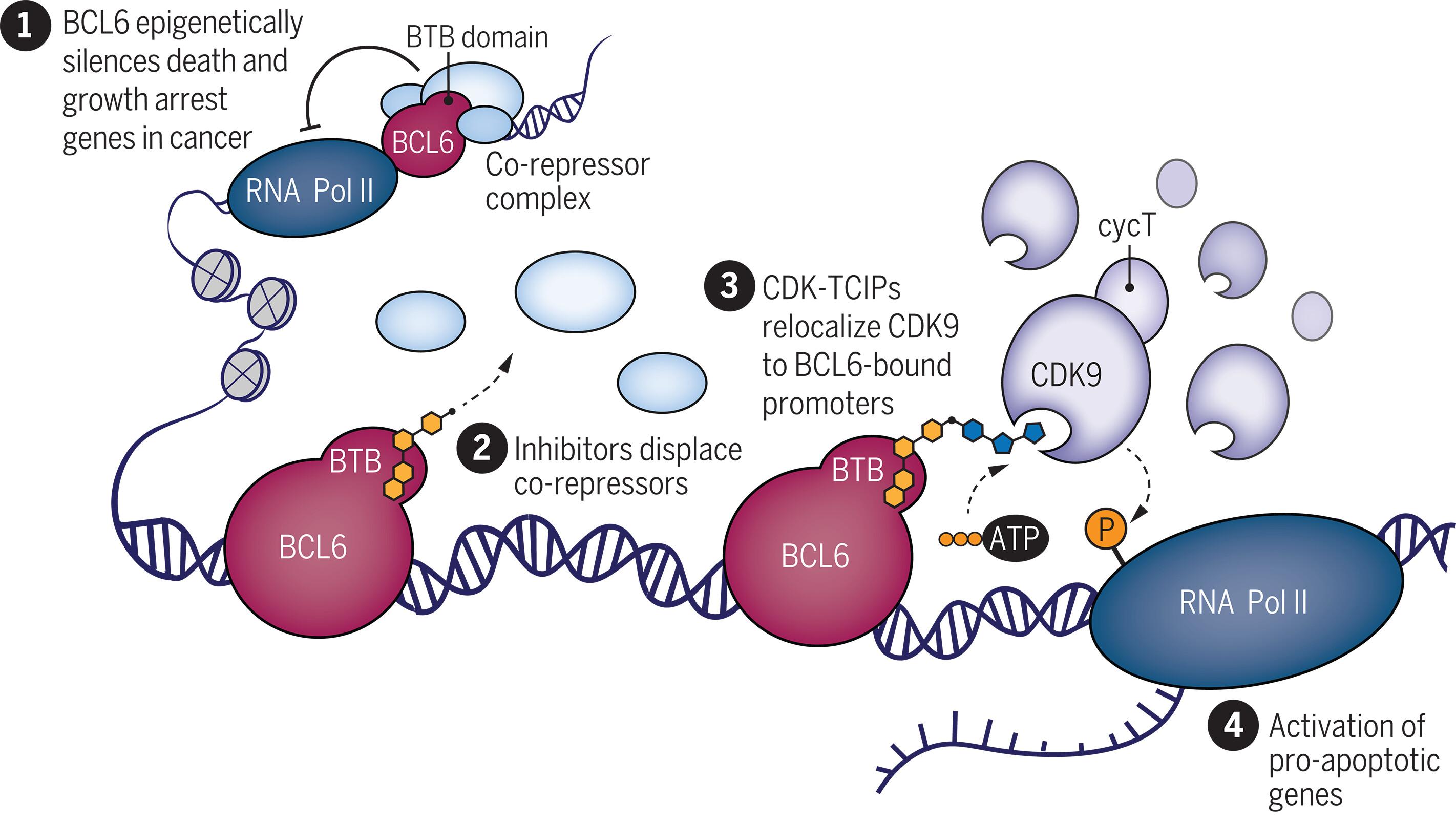
重新定位转录激酶,激活细胞凋亡。
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
激酶是细胞功能的关键调节因子,通常与疾病的发生机制有关。大多数针对激酶的药物都是抑制其催化活性的分子,但在这里,我们利用化学诱导的接近性将激酶抑制剂转化为治疗基因的激活剂。我们合成了二价分子,将转录因子 B 细胞淋巴瘤 6(BCL6)的配体与细胞周期蛋白依赖性激酶(CDK)的抑制剂连接起来。这些分子将 CDK9 重新定位到与 BCL6 结合的 DNA 上,并引导 RNA 聚合酶 II 发生磷酸化。由此导致的促凋亡BCL6靶基因的表达杀死了弥漫大B细胞淋巴瘤细胞,并特异性地消减了BCL6调控的生殖中心反应。基因组学和蛋白质组学证实了一种功能增益机制,其中全局激酶活性并未受到抑制,而是被重新定向。因此,激酶抑制剂可用于特异性激活转录。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Relocalizing transcriptional kinases to activate apoptosis
Kinases are critical regulators of cellular function that are commonly implicated in the mechanisms underlying disease. Most drugs that target kinases are molecules that inhibit their catalytic activity, but here we used chemically induced proximity to convert kinase inhibitors into activators of therapeutic genes. We synthesized bivalent molecules that link ligands of the transcription factor B cell lymphoma 6 (BCL6) to inhibitors of cyclin-dependent kinases (CDKs). These molecules relocalized CDK9 to BCL6-bound DNA and directed phosphorylation of RNA polymerase II. The resulting expression of pro-apoptotic, BCL6-target genes caused killing of diffuse large B cell lymphoma cells and specific ablation of the BCL6-regulated germinal center response. Genomics and proteomics corroborated a gain-of-function mechanism in which global kinase activity was not inhibited but rather redirected. Thus, kinase inhibitors can be used to context-specifically activate transcription.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


