对 crRNA 进行末端化学修饰可提高 CRISPR-Cas 在护理点核酸检测中的性能
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
CRISPR-Cas 系统通过 CRISPR RNA(crRNA)精确识别核酸,为在现场精确检测核酸带来了希望。然而,crRNA 本身易降解的特性给低资源环境下的精确检测带来了挑战。在这里,我们将化学修饰的 crRNA 用于基于 CRISPR-Cas 的检测(CM-CRISPR)。我们发现,对crRNA的延伸和化学修饰显著增强了LbCas12a的反式裂解活性。经化学修饰的crRNA具有抗降解性,CM-CRISPR在复杂环境中表现出卓越的检测能力。CM-CRISPR 可与重组酶聚合酶扩增(RPA)相结合,并应用于液滴数字平台,从而实现阿托摩尔级的灵敏度。我们还开发了一种用于数字 CRISPR 检测的便携式自动化设备,可用于床旁检测 (POCT)。免提取程序与该检测方法相结合,简化了工作流程,并成功检测了临床样本。这项研究找到了一种简单有效的方法来提高CRISPR-Cas的性能,并开发出了一种用于POCT的便携式平台,标志着基于CRISPR的诊断技术在实际应用中取得了重大进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
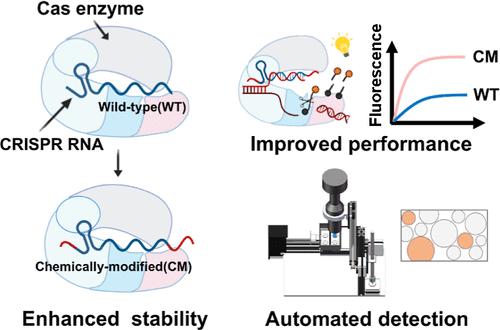
Terminal Chemical Modifications of crRNAs Enable Improvement in the Performance of CRISPR-Cas for Point-of-Care Nucleic Acid Detection
CRISPR-Cas systems, harnessing their precise nucleic acid recognition via CRISPR RNA (crRNA), offer promise for the accurate testing of nucleic acids in the field. However, the inherent susceptibility of crRNA to degradation poses challenges for accurate detection in low-resource settings. Here, we utilized the chemically modified crRNA for the CRISPR-Cas-based assay (CM-CRISPR). We found that the extension and chemical modification to crRNA significantly enhanced the trans-cleavage activity of LbCas12a. The chemically modified crRNA was resistant to degradation, and CM-CRISPR showed superior detection capability in complex environments. CM-CRISPR could be combined with recombinase polymerase amplification (RPA) and applied in a droplet digital platform, enabling attomolar-level sensitivity. We also developed a portable and automated device for a digital CRISPR assay, which is amenable to point-of-care testing (POCT). The extraction-free procedure was integrated with this assay to streamline the workflow, and clinical samples were successfully detected. This work finds a simple and efficient way to improve the performance of CRISPR-Cas and develops a portable platform for POCT, representing a significant advance toward practical applications of CRISPR-based diagnostics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


