蠕虫发育和活动试验(wDAT)作为发育危害化学筛选工具的优势和局限性
IF 3.3
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
蠕虫发育与活动测试(wDAT)测量 elegans 发育里程碑的获得时间和特定阶段的自发运动活动(SLA)。在此之前,wDAT 发现了哺乳动物发育毒物砷、铅和汞对 elegans 造成的发育延迟和 SLA 水平变化。5-氟尿嘧啶(5FU)、环磷酰胺(CP)、羟基脲(HU)和利巴韦林(RV)是致畸剂,也会诱发哺乳动物发育迟缓。至少在一些有关这些化学品的研究中,当哺乳动物的接触量低于致畸效应时,胎儿体重就会下降,这表明在小型替代整只动物模型中筛查发育迟缓可作为一般毒性终点,以确定需要进一步检测的化学品,从而检测是否会对发育产生更具体的不利影响。与哺乳动物的发育效应一致,5FU、HU 和 RV 与 wDAT 的发育迟缓有关。与发育迟缓相关的暴露会导致 5FU 和 HU 的活动能力低下,但 RV 会导致轻微的活动能力亢进。氯化石蜡是一种原药,需要细胞色素 P450s 的生物活化才能产生治疗和毒性作用。氯化石蜡在几种体外检测中都呈假阴性,在 wDAT 中也呈假阴性。这些结果表明,wDAT 有可能鉴别出某些发育毒物,如果某种未知物的 wDAT 结果呈阳性,则有必要在哺乳动物体内进行进一步检测。使用更大的阳性和阴性对照组进行进一步评估,将有助于确定这种线虫 wDAT 检测方法在毒性测试组或发育毒性评估证据权重法中的适用性和实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
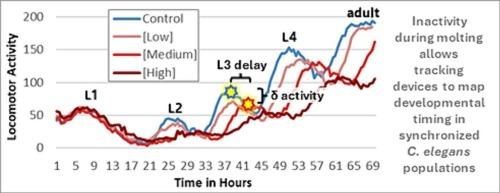
Strengths and limitations of the worm development and activity test (wDAT) as a chemical screening tool for developmental hazards
The worm Development and Activity Test (wDAT) measures C. elegans developmental milestone acquisition timing and stage-specific spontaneous locomotor activity (SLA). Previously, the wDAT identified developmental delays and SLA level changes in C. elegans with mammalian developmental toxicants arsenic, lead, and mercury. 5-fluorouracil (5FU), cyclophosphamide (CP), hydroxyurea (HU), and ribavirin (RV) are teratogens that also induce growth retardation in developing mammals. In at least some studies on each of these chemicals, fetal weight reductions were seen at mammalian exposures below those that had teratogenic effects, suggesting that screening for developmental delay in a small alternative whole-animal model could act as a general toxicity endpoint to identify chemicals for further testing for more specific adverse developmental outcomes. Consistent with mammalian developmental effects, 5FU, HU, and RV were associated with developmental delays with the wDAT. Exposures associated with developmental delay induced hypoactivity with 5FU and HU, but slight hyperactivity with RV. CP is a prodrug that requires bioactivation by cytochrome P450s for both therapeutic and toxic effects. CP tests as a false negative in several in vitro assays, and it was also a false negative with the wDAT. These results suggest that the wDAT has the potential to identify some developmental toxicants, and that a positive wDAT result with an unknown may warrant further testing in mammals. Further assessment with larger panels of positive and negative controls will help qualify the applicability and utility of this C. elegans wDAT assay within toxicity test batteries or weight of evidence approaches for developmental toxicity assessment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


