通过静电自组装制备纤维素基纳米颗粒,实现虾青素的 pH 值响应式输送
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
虾青素(AST)是一种强效抗氧化剂,但由于其溶解度、理化稳定性和生物利用度较低,其口服给药受到限制。本研究通过静电自组装 2,2,6,6-四甲基哌啶-1-酰氧基(TEMPO)氧化纤维素纳米纤维(TCNFs)和壳聚糖(CS),开发出 pH 响应型纳米载体,以增强虾青素的肠道递送。通过单因素实验和 Box-Behnken 设计对 TCNF/CS@AST 纳米颗粒进行了优化,克服了 AST 的疏水性,提高了其对环境应激的稳定性和在肠道环境中的可控释放性。透射电子显微镜证实,这些纳米颗粒的形状接近球形,平均流体力学直径为 64 纳米。TCNF/CS@AST 增强了 AST 消化后和在脂多糖刺激的 RAW 264.7 细胞中的抗氧化效果,同时显示出良好的细胞相容性。这些纳米颗粒为疏水性生物活性化合物的口服给药提供了一种前景广阔的策略,有望应用于精准营养领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
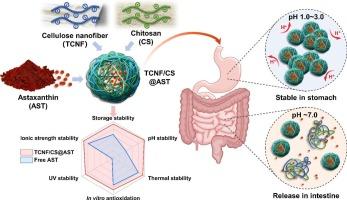
Preparation of cellulose-based nanoparticles via electrostatic self-assembly for the pH-responsive delivery of astaxanthin
Oral administration of astaxanthin (AST), a potent antioxidant, is limited owing to its low solubility, physicochemical stability, and bioavailability. This study developed pH-responsive nanocarriers by the electrostatic self-assembly of 2,2,6,6-tetramethylpiperidine-1-oxyradical (TEMPO)-oxidized cellulose nanofibers (TCNFs) and chitosan (CS) to enhance the intestinal delivery of AST. The TCNF/CS@AST nanoparticles were optimized through single-factor experiments and Box–Behnken design, subsequently overcoming the hydrophobicity of AST and demonstrating improved stability against environmental stressors and controlled release in the intestinal environment. Transmission electron microscopy confirmed the near-spherical shape of these nanoparticles, with an average hydrodynamic diameter of 64 nm. TCNF/CS@AST enhanced the antioxidant effectiveness of AST after digestion and in lipopolysaccharide-stimulated RAW 264.7 cells while demonstrating good cellular compatibility. These nanoparticles present a promising strategy for the oral delivery of hydrophobic bioactive compounds orally, with potential applications in precision nutrition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


