作为可持续植物蛋白新兴来源的草豌豆蛋白:结构、改性、功能和应用
IF 4.8
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
草豌豆是一种豆科作物,蛋白质含量在 20% 至 30% 之间,主要成分是约 66% 的球蛋白,以及谷蛋白(15%)、白蛋白(14%)和丙种球蛋白(5%)。草豌豆蛋白(GPP)成分通常采用碱性提取和酸沉淀法分离。在中性 pH 值下,GPP 成分的水溶性约为 60%。然而,这些成分的功能属性相对较差,限制了它们在众多食品和饮料产品中的应用。本综述介绍了作为植物蛋白新兴来源的禾本科豌豆蛋白,包括其结构、改性、功能及其在食品系统中的应用。"禾本科豌豆蛋白的功能属性可通过各种物理、化学和生物改性方法来提高,这些方法可改变蛋白质的构象、聚集或分子量。为此,人们采用了超声波、冷等离子体、热处理和高压处理等物理方法,以及蛋白质-多糖共轭和酶改性等化学方法。超声波等改性技术有可能使蛋白质的溶解度提高 90% 以上,并使 GPP 的乳化、发泡和凝胶特性显著提高两倍以上。GPP 的特性可通过多种分析方法进行表征,包括紫外可见光谱法、表面疏水性、游离巯基、傅里叶变换红外光谱法、圆二色法和十二烷基硫酸钠-聚丙烯酰胺凝胶电泳法。总之,由于具有良好的功能和营养特性,GPP 在食品工业中应用于植物性食品和饮料配方方面具有巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
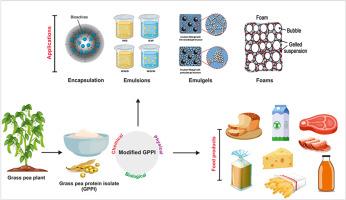
Grass pea protein as an emerging source of sustainable plant proteins: Structure, modification, functionality, and applications
Grass pea is a legume crop with a protein content ranging from 20% to 30%, primarily composed of approximately 66% globulin, along with glutelin (15%), albumin (14%), and prolamin (5%). Grass pea protein (GPP) ingredients are commonly isolated using alkaline extraction and acid precipitation methods. The water solubility of GPP ingredients is approximately 60% at neutral pH. However, the resulting functional attributes of these ingredients are relatively poor, which limits their application in numerous food and beverage products. This review describes the grass pea protein as an emerging source of plant proteins including structure, modification, functionality, as well as its applications in food systems.
The functional attributes of GPP can be enhanced using various physical, chemical, and biological modification methods that alter the conformation, aggregation, or molecular weight of the proteins. Physical methods like ultrasonication, cold plasma, heat treatment, and high-pressure treatment, as well as chemical methods like protein-polysaccharide conjugation and enzymatic modification, have been used for this purpose. Modification techniques such as ultrasonication has the potential to enhance protein solubility by over 90% and also significantly improve emulsifying, foaming, and gelation properties of GPPs by more than two-fold. The properties of GPPs can be characterized using a variety of analytical methods including UV–visible spectroscopy, surface hydrophobicity, free sulfhydryl groups, Fourier transform infrared spectroscopy, circular dichroism, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In conclusion, GPP holds great potential for application in the formulation of plant-based foods and beverages in the food industry due to its good functional and nutritional properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Bioscience
Biochemistry, Genetics and Molecular Biology-Biochemistry
CiteScore
6.40
自引率
5.80%
发文量
671
审稿时长
27 days
期刊介绍:
Food Bioscience is a peer-reviewed journal that aims to provide a forum for recent developments in the field of bio-related food research. The journal focuses on both fundamental and applied research worldwide, with special attention to ethnic and cultural aspects of food bioresearch.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


