pH 诱导界面蛋白结构变化以调整高压均质法制备的罗非鱼分离蛋白乳液的稳定性
IF 6.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
pH 值是影响蛋白质结构和乳化特性的关键外部因素。本研究旨在揭示高压均质法制备的罗非鱼蛋白分离物(TPI)在不同 pH 值(3.0-10.0)下二级结构变化与乳化稳定性之间的相关性。结果表明,当 pH 值远离等电点(pH 值为 5.0)时,TPI 的溶解度和乳化性能明显提高。同时,在 pH 值为 3.0 和 10.0 时,TPI 乳液的稳定性明显增强(粒径减小、zeta 电位增加、起皱指数接近 0、液滴分散均匀)。界面吸附蛋白主要由肌球蛋白重链和肌动蛋白组成,其二级结构受 pH 值和高压均质化的影响很大。当pH值接近pH 5.0时,α-螺旋会转变为β-片状结构和β-转折结构。然而,高压均质会诱导α-螺旋向β-片状转化。相关分析表明,乳液稳定性与α-螺旋呈正相关,而与β-片状呈负相关。这项研究深入揭示了二级结构变化与 TPI 乳液稳定性受 pH 值影响的相关性,为提高 TPI 乳液稳定性提供了另一种途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
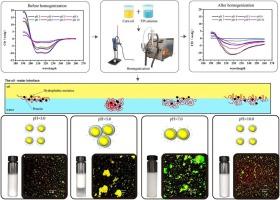
pH-induced interface protein structure changes to adjust the stability of tilapia protein isolate emulsion prepared by high-pressure homogenization
The pH is a crucial external factor affecting the structure and emulsification characteristics of proteins. The current study aimed to reveal the correlation between the secondary structure changes and tilapia protein isolate (TPI) emulsion stability under different pH (3.0–10.0) prepared by high-pressure homogenization. The results showed that TPI with significantly increased solubility and emulsifying properties when the pH keep away from the isoelectric point (pH 5.0). Meanwhile, TPI emulsions presented significantly enhanced stability (with decreased particle size, increased zeta potential, creaming index close to 0, and uniform dispersion of droplets) at pH 3.0 and 10.0. Interface-adsorbed protein mainly consists of a myosin-heavy chain and actin, and the secondary structure was significantly influenced by pH and high-pressure homogenization. The α-helix will be transformed into β-sheet and β-turn when pH is closer to pH 5.0. However, the high-pressure homogenization induced α-helix conversion to β-sheet. The correlation analysis revealed that emulsion stability is positively correlated with α-helix and negatively correlated with β-sheet. This work provides a deep insight into the correlation between secondary structure changes and the stability of TPI emulsion as affected by pH to offer an alternative way to enhance TPI emulsion stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry: X
CHEMISTRY, APPLIED-
CiteScore
4.90
自引率
6.60%
发文量
315
审稿时长
55 days
期刊介绍:
Food Chemistry: X, one of three Open Access companion journals to Food Chemistry, follows the same aims, scope, and peer-review process. It focuses on papers advancing food and biochemistry or analytical methods, prioritizing research novelty. Manuscript evaluation considers novelty, scientific rigor, field advancement, and reader interest. Excluded are studies on food molecular sciences or disease cure/prevention. Topics include food component chemistry, bioactives, processing effects, additives, contaminants, and analytical methods. The journal welcome Analytical Papers addressing food microbiology, sensory aspects, and more, emphasizing new methods with robust validation and applicability to diverse foods or regions.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


