甘草查尔酮 A 可通过减少真菌负荷和激活 Nrf2/HO-1 信号通路来改善烟曲霉角膜炎
IF 4
2区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
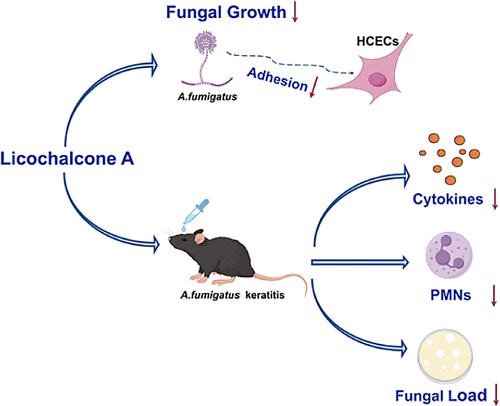
真菌性角膜炎(FK)是一种致盲性角膜感染性疾病。由于真菌入侵和宿主炎症反应过度,预后往往不佳。甘草查耳酮 A(Lico A)具有广泛的药理活性,包括抗真菌、抗炎、抗氧化和抗肿瘤特性。然而,人们尚未研究 Lico A 在 FK 中的作用。在这项研究中,我们发现 Lico A 可以破坏烟曲霉(A. fumigatus)的生物膜,抑制真菌生长和对宿主细胞的粘附,诱导菌丝形态的改变,并损害烟曲霉细胞膜和细胞壁的完整性以及线粒体结构。Lico A 可减轻小鼠 FK 的严重程度,减少中性粒细胞浸润和真菌负荷,并显著降低感染烟曲霉的小鼠角膜中的促炎细胞因子。在体外,我们也证实了 Lico A 能增加受烟曲霉菌刺激的人角膜上皮细胞(HCECs)核因子红细胞2相关因子2(Nrf2)和血红素加氧酶1(HO-1)在细胞核周围的表达。我们验证了 Lico A 的抗炎作用与 Nrf2/HO-1 轴的激活有关。这些结果表明,Lico A 可通过其抗炎和抗真菌活性在烟曲霉菌角膜炎中发挥保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Licochalcone A Ameliorates Aspergillus fumigatus Keratitis by Reducing Fungal Load and Activating the Nrf2/HO-1 Signaling Pathway
Fungal keratitis (FK) is a blinding corneal infectious disease. The prognosis is frequently unfavorable due to fungal invasion and an excessive host inflammatory response. Licochalcone A (Lico A) exhibits a broad spectrum of pharmacological activities, encompassing antifungal, anti-inflammatory, antioxidation, and antitumor properties. However, the role of Lico A has not yet been studied in FK. In this study, we discovered that Lico A could disrupt Aspergillus fumigatus (A. fumigatus) biofilms, inhibit fungal growth and adhesion to host cells, induce alterations of hyphal morphology, and impair the cell membrane and cell wall integrity and mitochondrial structure of A. fumigatus. Lico A can alleviate the severity of FK in mice, reduce neutrophil infiltration and fungal load, and significantly decrease the pro-inflammatory cytokines in mouse corneas infected with A. fumigatus. In vitro, we also demonstrated that Lico A increased the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) around the nucleus in human corneal epithelial cells (HCECs) stimulated with A. fumigatus. We verified that the anti-inflammatory effect of Lico A is associated with the activation of the Nrf2/HO-1 axis. These results indicated that Lico A could provide a protective role in A. fumigatus keratitis through its anti-inflammatory and antifungal activities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Infectious Diseases
CHEMISTRY, MEDICINALINFECTIOUS DISEASES&nb-INFECTIOUS DISEASES
CiteScore
9.70
自引率
3.80%
发文量
213
期刊介绍:
ACS Infectious Diseases will be the first journal to highlight chemistry and its role in this multidisciplinary and collaborative research area. The journal will cover a diverse array of topics including, but not limited to:
* Discovery and development of new antimicrobial agents — identified through target- or phenotypic-based approaches as well as compounds that induce synergy with antimicrobials.
* Characterization and validation of drug target or pathways — use of single target and genome-wide knockdown and knockouts, biochemical studies, structural biology, new technologies to facilitate characterization and prioritization of potential drug targets.
* Mechanism of drug resistance — fundamental research that advances our understanding of resistance; strategies to prevent resistance.
* Mechanisms of action — use of genetic, metabolomic, and activity- and affinity-based protein profiling to elucidate the mechanism of action of clinical and experimental antimicrobial agents.
* Host-pathogen interactions — tools for studying host-pathogen interactions, cellular biochemistry of hosts and pathogens, and molecular interactions of pathogens with host microbiota.
* Small molecule vaccine adjuvants for infectious disease.
* Viral and bacterial biochemistry and molecular biology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


