优化设计一种新型催化方法,利用介肠联白色念珠菌葡聚糖酶将葡萄糖基化的异麦芽寡糖转化为膳食多元醇结构
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
多元醇或糖醇在工业中被广泛用作甜味剂和食品配方配料,目的是防治与饮食相关的非传染性疾病。鉴于学术界和工业界对 "公认安全"(GRAS)酶的使用都很有吸引力,本研究报告了一种优化工艺,该工艺利用从介肠亮珠菌中提取的葡聚糖酶实现多元醇的转葡糖基化。这些酶的改性可产生具有异构寡糖(IMOS)结构的新一代葡糖基化多元醇,从而可能提供附加功能,如益生元效应。这些反应以实验设计框架为指导,旨在最大限度地提高潜在新型甜味剂的产量。在优化条件下,葡聚糖酸酶首先清除蔗糖的糖苷键,形成酶-葡糖共价中间复合物,释放出果糖。然后,受体底物(即多元醇)与酶-葡萄糖基中间体结合,导致葡萄糖基单元转移到受测多元醇中。通过核磁共振(NMR)和基质辅助激光解吸/电离飞行时间(MALDI-TOF)分析对反应产物的结构进行了深入研究,发现多元醇上存在线性α(1 → 6)糖苷键,从而产生了含有 4 到 10 个葡萄糖残基的寡糖结构。这些以多元醇为基础的新型低聚糖有望成为创新的益生甜味剂,为健康带来潜在的益处。本文章由计算机程序翻译,如有差异,请以英文原文为准。
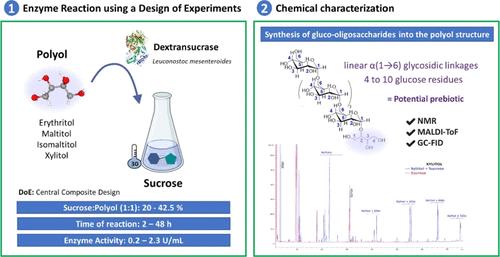
Design Optimization of a Novel Catalytic Approach for Transglucosylated Isomaltooligosaccharides into Dietary Polyols Structures by Leuconostoc mesenteroides Dextransucrase
Polyols, or sugar alcohols, are widely used in the industry as sweeteners and food formulation ingredients, aiming to combat the incidence of diet-related Non-Communicable Diseases. Given the attractive use of Generally Regarded As Safe (GRAS) enzymes in both academia and industry, this study reports on an optimized process to achieve polyols transglucosylation using a dextransucrase enzyme derived from Leuconostoc mesenteroides. These enzyme modifications could lead to the creation of a new generation of glucosylated polyols with isomalto-oligosaccharides (IMOS) structures, potentially offering added functionalities such as prebiotic effects. These reactions were guided by a design of experiment framework, aimed at maximizing the yields of potential new sweeteners. Under the optimized conditions, dextransucrase first cleared the glycosidic bond of sucrose, releasing fructose with the formation of an enzyme-glucosyl covalent intermediate complex. Then, the acceptor substrate (i.e., polyols) is bound to the enzyme-glucosyl intermediate, resulting in the transfer of glucosyl unit to the tested polyols. Structural insights into the reaction products were obtained through nuclear maneic resonance (NMR) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) analyses, which revealed the presence of linear α(1 → 6) glycosidic linkages attached to the polyols, yielding oligosaccharide structures containing from 4 to 10 glucose residues. These new polyols-based oligosaccharides hold promise as innovative prebiotic sweeteners, potentially offering valuable health benefits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


