探索杜冷丁类天然产物的非酶起源
IF 3.6
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
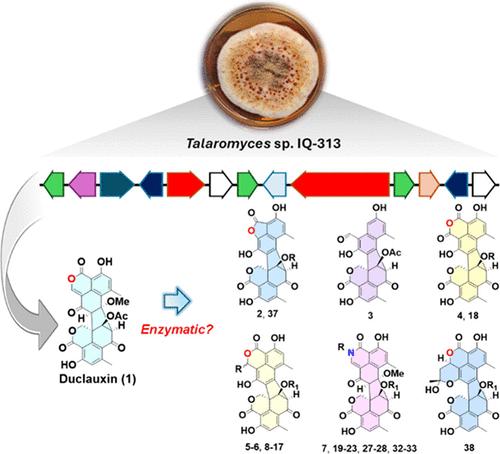
为增加用于药物化学目的的杜冷丁(1)类分子的多样性而进行的化学生物研究揭示了杜冷丁(1)对胺和醇的反应性。为了扩展该化合物类别,我们采用了将胺与杜克菌素 (1) 共轭的半合成策略。从这一方法中获得的启示使我们提出了一个假设,即某些类似杜克菌素的 "天然产物",如塔拉菌素 B (2)、枯草菌素 G (3)、异菌菌素 (4)、枯草菌素 F (5/6)、枯草菌素 J (8/9)、枯草菌素 I (12/13) 和疣菌素 A (38),可能是分离的人工产物,而不是酶的产物。进一步的实验涉及将 1 吸附到硅胶上,结果产生了 2-6。为了深入了解产生此类分子的条件,实验人员设置了温和条件下的一步反应。这两项实验的结果都证实,杜冷丁样分子是通过非酶促反应生成的。本文提出的分析证据表明,这些分子来源于 1,而作为主要中间体的是枯草杆菌素 J 的表聚混合物(8 和 9)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the Nonenzymatic Origin of Duclauxin-like Natural Products
Chemical-biological efforts to increase the diversity of duclauxin (1)-like molecules for medicinal chemistry purposes unveiled the reactivity of duclauxin (1) toward amines and alcohols. To expand the compound class, a semisynthetic strategy conjugating amines to duclauxin (1) was employed. Insights gained from this approach led to the hypothesis that certain duclauxin-like “natural products” such as talaromycesone B (2), bacillisporin G (3), xenoclauxin (4), bacillisporins F (5/6), bacillisporins J (8/9), bacillisporins I (12/13), and verruculosin A (38) may be isolation artifacts rather than enzymatic products. Further experimentation, involving adsorption of 1 onto silica gel, resulted in the production of 2–6. To gain insights into the conditions that generate such molecules, one-step reactions under mild conditions were set. Outcomes from both experiments confirmed that duclauxin-like molecules are generated via nonenzymatic reactions. This article presents analytical evidence, indicating that these molecules originate from 1, with the epimeric mixture of bacillisporins J (8 and 9) acting as the primary intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


