二聚植物大麻素 Cannabitwinol(CBD 二聚体)及其类似物的全合成。
IF 3.3
2区 生物学
Q2 CHEMISTRY, MEDICINAL
Journal of Natural Products
Pub Date : 2024-09-27
Epub Date: 2024-08-26
DOI:10.1021/acs.jnatprod.4c00674
引用次数: 0
摘要
本研究揭示了一种从市售原料中获取天然产品植物大麻素 cannabitwinol (1) 的新合成路线。我们还展示了利用本序列合成其他相关二聚体(包括其对映体)的方法。这条路线避免了有关获取大麻植物材料或 CBD 的法律限制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
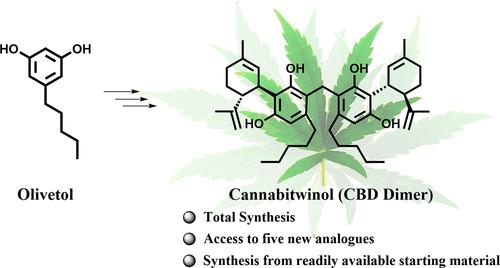
Total Synthesis of the Dimeric Phytocannabinoid Cannabitwinol (CBD Dimer) and Its Analogues.
A new synthetic route to access the natural product phytocannabinoid cannabitwinol (1) starting from commercially available raw materials is disclosed. We have also demonstrated the synthesis of other related dimers, including their enantiomers, using the present sequence. This route avoids the legal constraints concerning the acquisition of cannabis plant material or CBD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


