从源于土壤的真菌 Clonostachys rosea YRS-06 中获得的生物碱 Clonorosin B 的修正结构合成。
IF 3.6
2区 生物学
Q2 CHEMISTRY, MEDICINAL
Journal of Natural Products
Pub Date : 2024-09-27
Epub Date: 2024-08-20
DOI:10.1021/acs.jnatprod.4c00777
引用次数: 0
摘要
通过化学合成法,从 2,3-二甲氧基苯甲醛开始,经过中间体 8、12 和 14,经过六个简单步骤,制备出了标题天然产物的修正结构 2。将合成得到的化合物 2 的核磁共振数据与报告的天然产物的核磁共振数据进行比较,发现两者非常吻合。对化合物 2 以及类似物/前体 7、9、10、11、13、14 和 15 进行初步生物筛选后发现,它们都不具有抗菌、抗真菌或细胞毒性作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
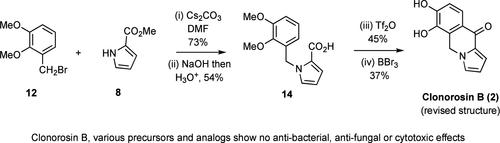
Synthesis of the Corrected Structure Assigned to Clonorosin B, an Alkaloid Obtained from the Soil-derived Fungus Clonostachys rosea YRS-06.
The revised structure, 2, assigned to the title natural product has been prepared by chemical synthesis using a reaction sequence involving six simple steps starting from 2,3-dimethoxybenzaldehyde and proceeding via intermediates 8, 12, and 14. A comparison of the NMR data acquired on synthetically derived compound 2 with those reported for the natural product reveals an excellent match. Preliminary biological screening of compound 2 along with analogues/precursors 7, 9, 10, 11, 13, 14, and 15 revealed that none exhibited antibacterial, antifungal or cytotoxic effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


