Drymariamides A-J, Antiadipogenic Cyclopeptides Incorporating Noncanonical Amino Acid from Drymaria cordata.
IF 3.6
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
在此,我们报告了一项针对旱莲草全株的广泛植物化学研究,该研究分离出了十种新的眶苷类化合物,命名为旱莲草酰胺 A-J(1-10)。化合物 2、3 和 5 包含犬尿氨酸(Kyn)或 3a-羟基吡咯吲哚啉(HPI)的非典型氨基酸的稀有残基。通过结合光谱分析、先进的马菲法、X 射线衍射和电子圆二色性分析,阐明了它们的绝对构型结构。化合物 1-10 在 3T3-L1 脂肪细胞中表现出抗脂肪生成作用,其中最有效的化合物 7 的 EC50 值为 1.17 ± 0.19 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
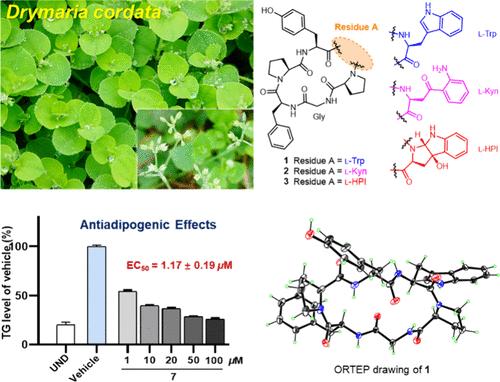
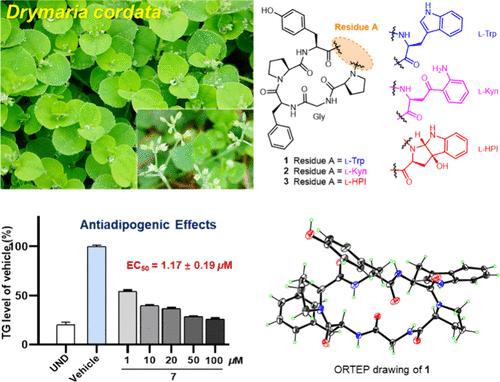
Drymariamides A–J, Antiadipogenic Cyclopeptides Incorporating Noncanonical Amino Acids from Drymaria cordata
Herein, we report an extensive phytochemical study on the whole plant of Drymaria cordata, which led to the isolation of ten new orbitides, named drymariamides A–J (1–10). Compounds 2, 3, and 5 incorporate rare residues of noncanonical amino acids of kynurenine (Kyn) or 3a-hydroxypyrroloindoline (HPI). Their structures with absolute configurations were elucidated by a combination of spectroscopic analysis, advanced Marfey’s method, X-ray diffraction, and electronic circular dichroism analysis. Compounds 1–10 exhibited antiadipogenic effects in 3T3-L1 adipocytes, and the most potent compound 7 showed an EC50 value of 1.17 ± 0.19 μM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


