马氏反应影响栉水母食物基质中肌球蛋白的免疫结合活性和消化率
IF 7.4
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
近年来,牡蛎过敏率激增,日常食品加工方法难以降低耐热性和消化道过敏性,如滋养肌肽(TM)。在这项研究中,木糖(Xyl)的马氏反应显著降低了Alectryonella plicatula食物基质(AFM)的IgE结合能力,降低了(77.81 ± 2.68)%。研究发现,马氏反应改变了 AFM 的结构,其中 α-螺旋的含量减少了(24.64 ± 1.46)%。结构转变进一步解释了为什么马氏反应会改变 AFM 的免疫结合活性。此外,马氏反应降低了 AFM 的消化稳定性,使 A. plicatula 食物基质马氏反应产物(AFM-MRPs)中的 TM 更容易被消化。根据上述研究,TM 的 7 个 IgE 表位上有 10 个氨基酸被修饰。这一结果表明,马氏反应通过改变结构和修饰表位上的氨基酸,降低了 AFM 的免疫结合活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
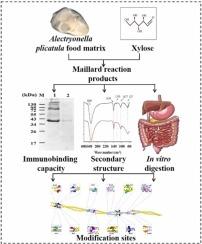
Maillard reaction affecting immunobinding activity and digestibility of tropomyosin in Alectryonella plicatula food matrix
In recent years, the allergy rate of oysters has surged, and daily food processing methods make it hard to reduce heat resistance and digestive allergy such as tropomyosin (TM). In this study, the Maillard reaction with xylose (Xyl) significantly reduced the IgE binding capacity of Alectryonella plicatula food matrix (AFM), that reduced by (77.81 ± 2.68)%. The study found the Maillard reaction changes the structure of the AFM, in which the content of α-helix decreased by (24.64 ± 1.46)%. Structural transformation further explains why the Maillard reaction alters the immunobinding activity of AFM. In addition, the Maillard reaction reduces the digestive stability of the AFM and makes TM in the A. plicatula food matrix Maillard reaction products (AFM-MRPs) more easily digested. Based on the above research, 10 amino acids on the 7 IgE epitopes of TM were modified. This result indicates that the Maillard reaction reduces the immunobinding activity of the AFM by changing the structure and modifying the amino acids on the epitope.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Science and Human Wellness
Agricultural and Biological Sciences-Food Science
CiteScore
8.30
自引率
5.70%
发文量
80
审稿时长
28 days
期刊介绍:
Food Science and Human Wellness is an international peer-reviewed journal that provides a forum for the dissemination of the latest scientific results in food science, nutriology, immunology and cross-field research. Articles must present information that is novel, has high impact and interest, and is of high scientific quality. By their effort, it has been developed to promote the public awareness on diet, advocate healthy diet, reduce the harm caused by unreasonable dietary habit, and directs healthy food development for food industrial producers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


