2′-岩藻糖通过重塑肠道微生物群和激活AHR通路缓解免疫检查点阻断剂相关性结肠炎
IF 7.4
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
免疫检查点阻断(ICB)疗法在癌症免疫疗法中非常有效,但胃肠道毒性限制了其应用。肠道微生物群在 ICB 相关性结肠炎中起着至关重要的作用。2′-岩藻酸半乳糖(2′FL)是母乳中含量最丰富的益生元,可重塑肠道微生物群并发挥免疫调节作用。本研究旨在确定2′FL对ICB相关性结肠炎的影响,并揭示其介导机制。伊匹单抗和葡聚糖硫酸钠诱导了ICB相关性结肠炎。口服2′FL(0.6克/(千克/天))通过增强调节性T细胞(Treg)和结肠中巨噬细胞的M2/M1比值来改善ICB诱导的结肠炎。2′FL还能增加紧密连接蛋白(Zonula occludens-1(ZO-1)和粘蛋白2(MUC2))和抗氧化应激指标(超氧化物歧化酶(SOD)和过氧化氢酶(CAT))的表达。此外,2′FL 还能增加双歧杆菌和乳酸杆菌的数量,提高微生物代谢产物(如吲哚-3-乳酸(ILA))的水平,从而激活芳基烃受体配体(AHR)途径。消耗肠道微生物群后,2′FL 的保护作用消失,而 ILA 处理可部分模拟 2′FL 的保护作用。值得注意的是,2′FL 对抗肿瘤免疫没有抑制作用。这些研究结果表明,2′FL 可以通过调节肠道微生物群和细菌代谢产物作为一种潜在的保护性策略来治疗 ICB 相关性结肠炎。本文章由计算机程序翻译,如有差异,请以英文原文为准。
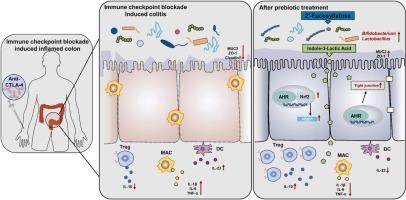
2′-Fucosyllactose alleviate immune checkpoint blockade-associated colitis by reshaping gut microbiota and activating AHR pathway
Immune checkpoint blockade (ICB) therapeutics are highly effective in cancer immunotherapy, but gastrointestinal toxicity limited the application. Intestinal microbiota plays a crucial role in ICB-associated colitis. 2′-Fucosyllactose (2′FL) is most abundance prebiotic in human milk that can reshape gut microbiota and exert immune regulatory effect. The study aimed to determine the effects of 2′FL on ICB-associated colitis and to uncover the mediating mechanism. ICB-associated colitis was induced by the ipilimumab and dextran sulfate sodium. Oral administration of 2′FL (0.6 g/(kg∙day)) ameliorated ICB-induced colitis by enhancing regulatory T cells (Treg) and the M2/M1 ratio of macrophages in colon. 2′FL treatment also increased the expression of tight junction proteins (zonula occludens-1 (ZO-1) and mucin 2 (MUC2)) and antioxidant stress indicators (superoxide dismutase (SOD) and catalase (CAT)). In addition, administration of 2′FL increased the abundance of Bifidobacterium and Lactobacillus, and elevated the levels of microbial metabolites, such as indole-3-lactic acid (ILA), which activated the aryl hydrocarbon receptor ligands (AHR) pathway. The protective effect of 2′FL was abolished upon depletion of gut microbiota, and ILA treatment partially simulated the protective effect of 2′FL. Notably, 2′FL did not exhibit inhibition of antitumor immunity. These findings suggest that 2′FL could serve as a potential protective strategy for ICB-associated colitis by modulating the intestinal microbiota and bacterial metabolites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Science and Human Wellness
Agricultural and Biological Sciences-Food Science
CiteScore
8.30
自引率
5.70%
发文量
80
审稿时长
28 days
期刊介绍:
Food Science and Human Wellness is an international peer-reviewed journal that provides a forum for the dissemination of the latest scientific results in food science, nutriology, immunology and cross-field research. Articles must present information that is novel, has high impact and interest, and is of high scientific quality. By their effort, it has been developed to promote the public awareness on diet, advocate healthy diet, reduce the harm caused by unreasonable dietary habit, and directs healthy food development for food industrial producers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


