Inhibiting bridge integrator 2 phosphorylation leads to improved oocyte quality, ovarian health and fertility in aging and after chemotherapy in mice
IF 19.4
Q1 CELL BIOLOGY
引用次数: 3
Abstract
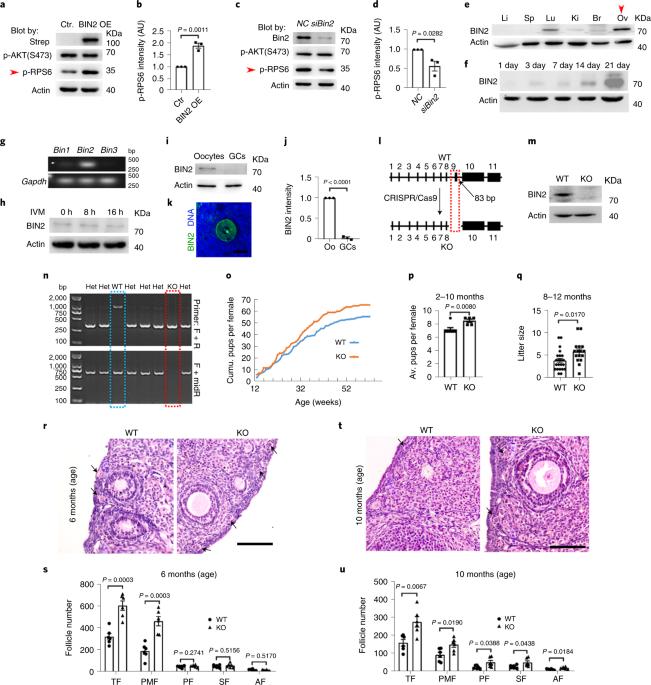
Female ovaries degenerate about 20 years earlier than testes leading to reduced primordial follicle reserve and a reduction in oocyte quality. Here we found that bridge integrator 2 (BIN2) is enriched in mouse ovaries and oocytes and that global knockout of this protein improves both female fertility and oocyte quality. Quantitative ovarian proteomics and phosphoproteomics showed that Bin2 knockout led to a decrease in phosphorylated ribosomal protein S6 (p-RPS6), a component of the mammalian target of rapamycin pathway and greatly increased nicotinamide nucleotide transhydrogenase (NNT), the free-radical detoxifier. Mechanistically, we find that phosphorylation of BIN2 at Thr423 and Ser424 leads to its translocation from the membrane to the cytoplasm, subsequent phosphorylation of RPS6 and inhibition of Nnt translation. We synthesized a BIN2-penetrating peptide (BPP) designed to inhibit BIN2 phosphorylation and found that a 3-week BPP treatment improved primordial follicle reserve and oocyte quality in aging and after chemotherapy-induced premature ovarian failure without discernible side effects. The authors found that deleting an ovary-rich gene called Bin2 improved fertility and oocyte quality in mice. Inhibiting BIN2 with a cell-penetrating peptide improved ovarian function in aging and in chemotherapy-treated mice.
抑制桥接整合子 2 磷酸化可改善衰老和化疗后小鼠的卵母细胞质量、卵巢健康和生育能力
雌性卵巢退化比睾丸退化早约 20 年,导致原始卵泡储备减少和卵母细胞质量下降。在这里,我们发现桥整合子 2(BIN2)富集于小鼠卵巢和卵母细胞中,全面敲除该蛋白可提高雌性生育能力和卵母细胞质量。定量卵巢蛋白质组学和磷酸化蛋白质组学显示,Bin2基因敲除导致磷酸化核糖体蛋白S6(p-RPS6)(哺乳动物雷帕霉素靶蛋白通路的一个组成部分)减少,并大大增加了烟酰胺核苷酸转氢酶(NNT)(自由基解毒剂)。从机理上讲,我们发现 BIN2 在 Thr423 和 Ser424 处的磷酸化导致其从膜转位到细胞质,随后 RPS6 磷酸化并抑制 Nnt 翻译。我们合成了一种旨在抑制 BIN2 磷酸化的 BIN2 穿透肽(BPP),并发现为期 3 周的 BPP 治疗可改善衰老和化疗引起的卵巢早衰后的原始卵泡储备和卵母细胞质量,且无明显副作用。作者发现,删除一种名为 Bin2 的卵巢丰富基因可提高小鼠的生育能力和卵母细胞质量。用一种细胞穿透肽抑制BIN2可改善衰老和化疗小鼠的卵巢功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


