Copper induces cell death by targeting lipoylated TCA cycle proteins
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 1017
Abstract
Copper is an essential cofactor for all organisms, and yet it becomes toxic if concentrations exceed a threshold maintained by evolutionarily conserved homeostatic mechanisms. How excess copper induces cell death, however, is unknown. Here, we show in human cells that copper-dependent, regulated cell death is distinct from known death mechanisms and is dependent on mitochondrial respiration. We show that copper-dependent death occurs by means of direct binding of copper to lipoylated components of the tricarboxylic acid (TCA) cycle. This results in lipoylated protein aggregation and subsequent iron-sulfur cluster protein loss, which leads to proteotoxic stress and ultimately cell death. These findings may explain the need for ancient copper homeostatic mechanisms.
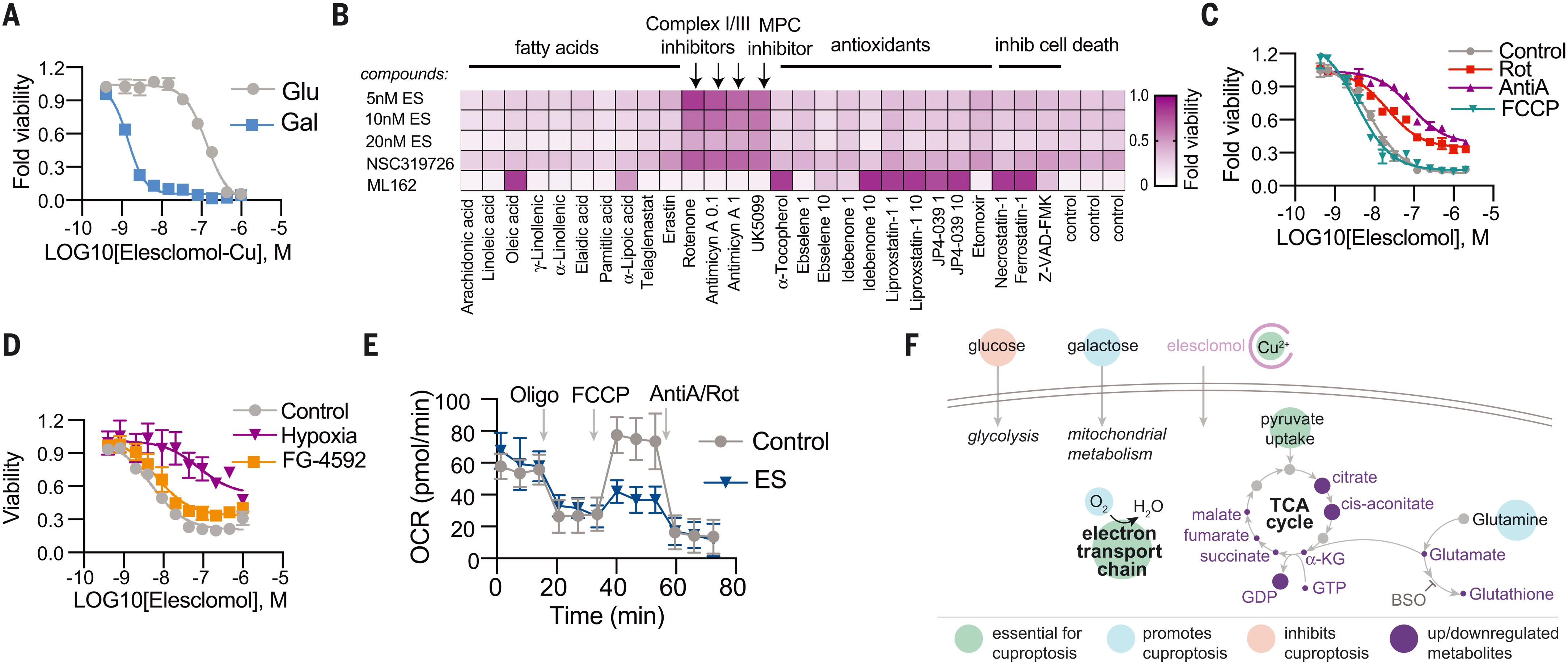
铜通过靶向脂酰化 TCA 循环蛋白诱导细胞死亡
铜是所有生物体内不可或缺的辅助因子,然而,如果铜的浓度超过了由进化保守的平衡机制维持的阈值,铜就会变成有毒物质。然而,过量的铜如何诱导细胞死亡尚不清楚。在这里,我们在人体细胞中发现,依赖铜的调节性细胞死亡不同于已知的死亡机制,它依赖于线粒体呼吸。我们发现,铜依赖性死亡是通过铜与三羧酸(TCA)循环中的脂酰化成分直接结合而发生的。这导致脂酰化蛋白质聚集和随后的铁硫簇蛋白质丢失,从而导致蛋白质毒性应激,最终导致细胞死亡。这些发现可以解释为什么需要古老的铜平衡机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


