α-Pyrone Derivatives from Calcarisporium arbuscula Discovered by Genome Mining
IF 3.3
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
A highly reducing polyketide synthase (HRPKS) gene cluster from the genome of Calcarisporium arbuscula was identified through genome mining. Heterologous expression of this cluster led to the production of four new α-pyrone compounds, calcapyrones A (1) and B (2), along with their biosynthetic intermediates calcapyrones C (3) and D (4). The structures of these compounds were elucidated on the basis of extensive spectroscopic experiments, and the absolute configurations of the 7,8-diol moieties in 1 and 2 were assigned using Snatzke’s method. The biosynthetic pathway of 1 and 2 was established through in vivo and in vitro experiments.
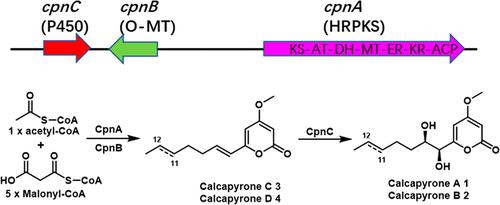
利用基因组挖掘技术从熊果木中发现α-Pyrone衍生物。
通过基因组挖掘,从熊果木基因组中鉴定出一个高还原性聚酮合酶(HRPKS)基因簇。该簇的异源表达导致产生了四种新的α-吡喃酮化合物,钙吡咯烷A(1)和B(2),以及它们的生物合成中间体钙吡咯烷C(3)和D(4)。这些化合物的结构是在广泛的光谱实验的基础上阐明的,并且使用Snatzke的方法分配了1和2中7,8-二醇部分的绝对构型。通过体内和体外实验建立了1和2的生物合成途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


