3′-O-β-Glucosyl-4′,5′-didehydro-5′-deoxyadenosine Is a Natural Product of the Nucleocidin Producers Streptomyces virens and Streptomyces calvus
IF 3.3
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
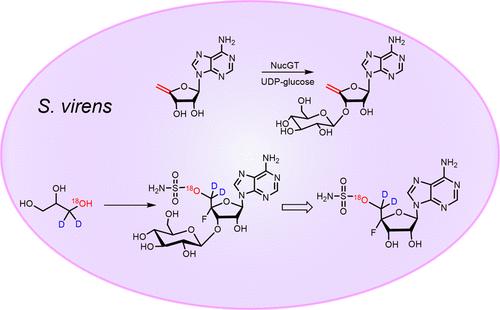
3′-O-β-Glucosyl-4′,5′-didehydro-5′-deoxyadenosine 13 is identified as a natural product of Streptomyces calvus and Streptomyces virens. It is also generated in vitro by direct β-glucosylation of 4′,5′-didehydro-5′-deoxyadenosine 12 with the enzyme NucGT. The intact incorporation of oxygen-18 and deuterium isotopes from (±)[1-18O,1-2H2]-glycerol 14 into C-5′ of nucleocidin 1 and its related metabolites precludes 3′-O-β-glucosyl-4′,5′-didehydro-5′-deoxyadenosine 13 as a biosynthetic precursor to nucleocidin 1.
3′-O-β-葡糖基-4′,5′-二氢-5′-脱氧腺苷是核糖核酸酶产生菌病毒链霉菌和卡尔维斯链霉菌的天然产物
3′-O-β-葡糖基-4′,5′-二氢-5′-脱氧腺苷13是裂壳链霉菌和病毒链霉菌的天然产物。它也是通过4′,5′-二脱氢-5′-脱氧腺苷12与NucGT酶的直接β-葡糖基化在体外产生的。来自(±)[1-18O,1-2H2]-甘油14的氧-18和氘同位素完整地结合到核调素1的C-5′及其相关代谢产物中,排除了3′-O-β-葡糖基-4′,5′-二氢-5′-脱氧腺苷13作为核调素的生物合成前体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


