Energy metabolism in mammalian sperm motility.
IF 4.9
3区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 17
Abstract
Mammalian sperm, the only cells that achieve their purpose outside their organism of origin, have to swim vigorously within the female reproductive tract to reach an oocyte. Flagellar dyneins drive sperm motility, which accounts for the consumption of high amounts of ATP. The two main ATP-producing metabolic pathways are compartmentalized in sperm: oxidative phosphorylation in the midpiece and glycolysis in the principal piece. The relative preponderance of these pathways has been discussed for decades (the so-called sperm energy debate). The debate has been muddled by species-specific variances and by technical constraints. But recent findings suggest that sperm from most mammalian species employ a versatile metabolic strategy to maintain motility according to the physiological environment. Different metabolic pathways likely coordinate by using exogenous and/or endogenous substrates in order to produce ATP efficiently. Defects in any of these pathways (glycolysis, mitochondrial oxidative phosphorylation, Krebs cycle, fatty acids oxidation, and ketone bodies oxidation, among others) may disturb sperm motility and be at the origin of male infertility. Understanding sperm bioenergetics is thus crucial for building new diagnostic tools, and for the development of treatments for patients presenting with low sperm motility. Some of these patients may benefit from personalized metabolic supplementations and dietary interventions. This article is categorized under: Reproductive System Diseases > Molecular and Cellular Physiology.
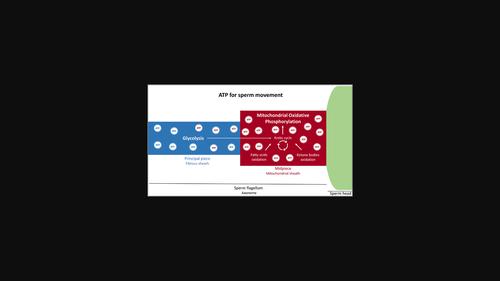
哺乳动物精子运动中的能量代谢。
哺乳动物精子是唯一在其起源生物体外达到其目的的细胞,必须在雌性生殖道内剧烈游动才能到达卵母细胞。鞭毛动力蛋白驱动精子运动,这是消耗大量ATP的原因。精子中产生ATP的两种主要代谢途径是分区的:中段的氧化磷酸化和主要部分的糖酵解。这些途径的相对优势已经讨论了几十年(所谓的精子能量辩论)。由于物种差异和技术限制,这场争论一直被搅乱。但最近的研究结果表明,大多数哺乳动物的精子根据生理环境采用多种代谢策略来保持活力。不同的代谢途径可能通过使用外源和/或内源性底物来协调,以便有效地产生ATP。这些途径中的任何一种(糖酵解、线粒体氧化磷酸化、克雷布斯循环、脂肪酸氧化和酮体氧化等)的缺陷都可能干扰精子运动,是男性不育的根源。因此,了解精子生物能量学对于构建新的诊断工具和开发精子活力低下患者的治疗方法至关重要。其中一些患者可能受益于个性化的代谢补充和饮食干预。本文分类在:生殖系统疾病>分子和细胞生理学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

WIREs Mechanisms of Disease
MEDICINE, RESEARCH & EXPERIMENTAL-
CiteScore
11.40
自引率
0.00%
发文量
45
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


