Expression of the gene encoding blood coagulation factor VIII without domain B in E. coli bacterial expression system.
引用次数: 0
Abstract
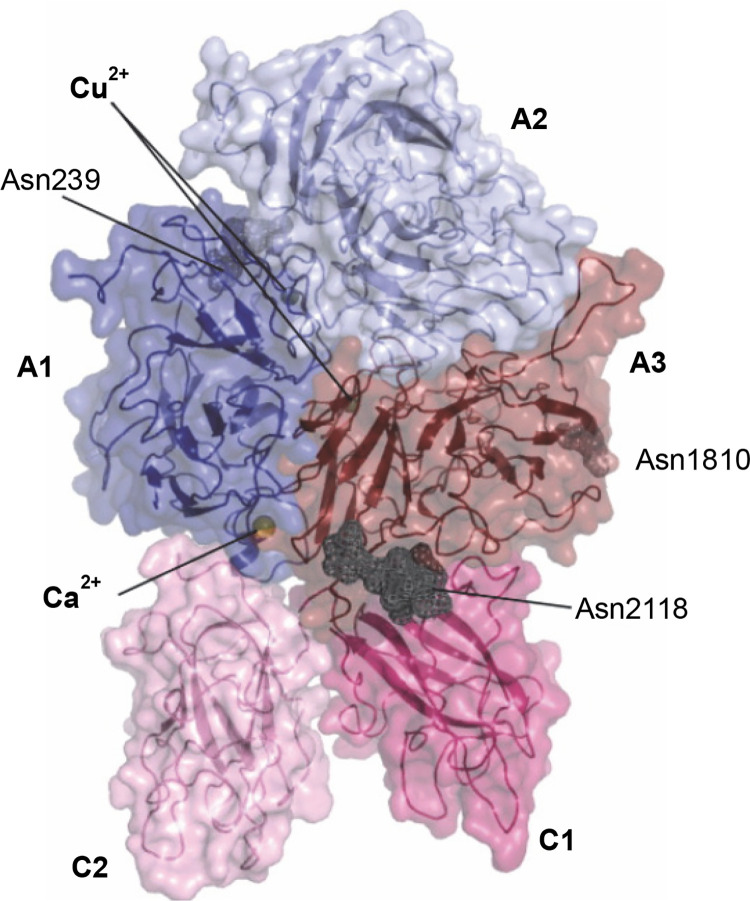
In this article, we have demonstrated the feasibility of generating an active form of recombinant blood coagulation factor VIII using an E. coli bacterial expression system as a potential treatment for hemophilia type A. Factor VIII (FVIII), an essential blood coagulation protein, is a key component of the fluid phase blood coagulation system. So far, all available recombinant FVIII formulations have been produced using eukaryotic expression systems. Mammalian cells can produce catalytically active proteins with all the necessary posttranslational modifications. However, cultivating such cells is time-consuming and highly expensive, and the amount of the obtained product is usually low. In contrast to eukaryotic cells, bacterial culture is inexpensive and allows the acquisition of large quantities of recombinant proteins in a short time. With this study, we aimed to obtain recombinant blood coagulation factor VIII using the E. coli bacterial expression system, a method not previously explored for this purpose. Our research encompasses the synthesis of blood coagulation factor VIII and its expression in a prokaryotic system. To achieve this, we constructed a prokaryotic expression vector containing a synthetic factor VIII gene, which was then used for the transformation of an E. coli bacterial strain. The protein expression was confirmed by mass spectrometry, and we assessed the stability of the gene construct while determining the optimal growth conditions. The production of blood coagulation factor VIII by the E. coli bacterial strain was carried out on a quarter-technical scale. We established the conditions for isolation, denaturation, and renaturation of the protein, and subsequently confirmed the activity of FVIII.

编码无结构域B的凝血因子VIII的基因在大肠杆菌细菌表达系统中的表达。
在这篇文章中,我们已经证明了使用大肠杆菌细菌表达系统产生活性形式的重组凝血因子VIII作为a型血友病的潜在治疗方法的可行性。因子VIII(FVIII)是一种必需的凝血蛋白,是液相凝血系统的关键组分。到目前为止,所有可用的重组FVIII制剂都是使用真核表达系统生产的。哺乳动物细胞可以产生具有所有必要翻译后修饰的催化活性蛋白质。然而,培养这样的细胞是耗时且高成本的,并且所获得的产物的量通常较低。与真核细胞相比,细菌培养成本低廉,可以在短时间内获得大量重组蛋白。在这项研究中,我们旨在使用大肠杆菌表达系统获得重组凝血因子VIII,这是一种以前没有探索过的方法。我们的研究包括凝血因子VIII的合成及其在原核系统中的表达。为了实现这一点,我们构建了含有合成因子VIII基因的原核表达载体,然后将其用于转化大肠杆菌菌株。蛋白质表达通过质谱法得到证实,我们在确定最佳生长条件的同时评估了基因构建体的稳定性。由大肠杆菌菌株生产凝血因子VIII是在四分之一的技术规模上进行的。我们建立了分离、变性和复性蛋白质的条件,随后证实了FVIII的活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


