Targeting metabolic vulnerabilities to overcome resistance to therapy in acute myeloid leukemia.
IF 4.6
Q1 ONCOLOGY
引用次数: 0
Abstract
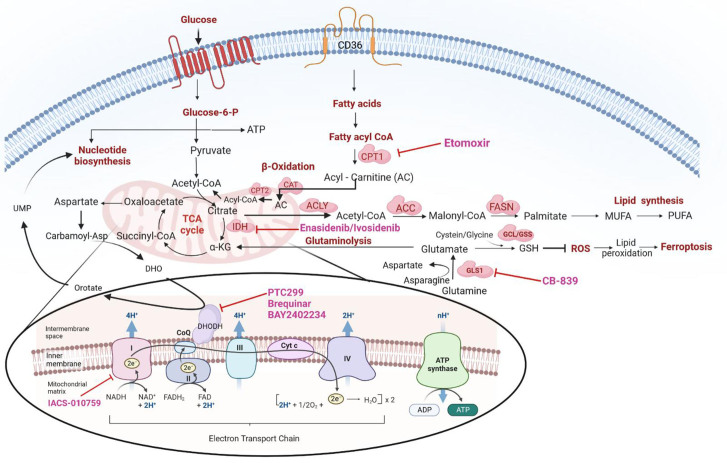
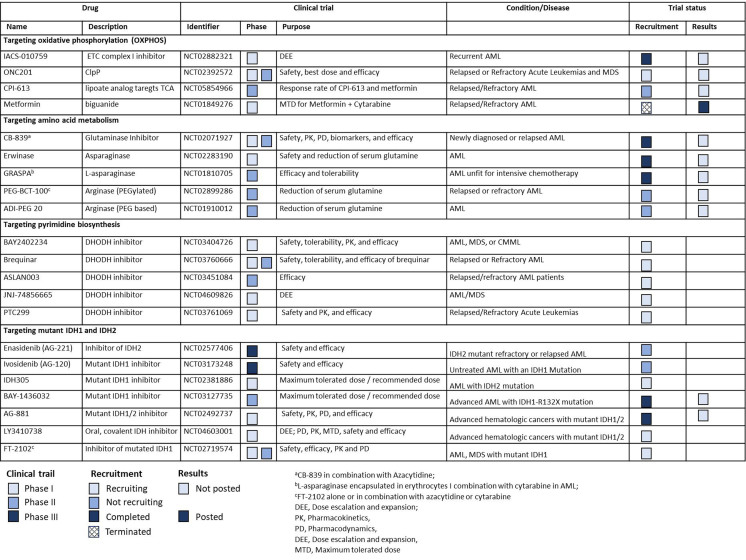
Malignant hematopoietic cells gain metabolic plasticity, reorganize anabolic mechanisms to improve anabolic output and prevent oxidative damage, and bypass cell cycle checkpoints, eventually outcompeting normal hematopoietic cells. Current therapeutic strategies of acute myeloid leukemia (AML) are based on prognostic stratification that includes mutation profile as the closest surrogate to disease biology. Clinical efficacy of targeted therapies, e.g., agents targeting mutant FMS-like tyrosine kinase 3 (FLT3) and isocitrate dehydrogenase 1 or 2, are mostly limited to the presence of relevant mutations. Recent studies have not only demonstrated that specific mutations in AML create metabolic vulnerabilities but also highlighted the efficacy of targeting metabolic vulnerabilities in combination with inhibitors of these mutations. Therefore, delineating the functional relationships between genetic stratification, metabolic dependencies, and response to specific inhibitors of these vulnerabilities is crucial for identifying more effective therapeutic regimens, understanding resistance mechanisms, and identifying early response markers, ultimately improving the likelihood of cure. In addition, metabolic changes occurring in the tumor microenvironment have also been reported as therapeutic targets. The metabolic profiles of leukemia stem cells (LSCs) differ, and relapsed/refractory LSCs switch to alternative metabolic pathways, fueling oxidative phosphorylation (OXPHOS), rendering them therapeutically resistant. In this review, we discuss the role of cancer metabolic pathways that contribute to the metabolic plasticity of AML and confer resistance to standard therapy; we also highlight the latest promising developments in the field in translating these important findings to the clinic and discuss the tumor microenvironment that supports metabolic plasticity and interplay with AML cells.
针对代谢脆弱性克服急性髓系白血病治疗耐药性。
恶性造血细胞获得代谢可塑性,重组合成代谢机制以提高合成代谢输出并防止氧化损伤,绕过细胞周期检查点,最终击败正常造血细胞。目前急性髓细胞白血病(AML)的治疗策略是基于预后分层,包括突变谱作为最接近疾病生物学的替代品。靶向治疗的临床疗效,例如靶向突变FMS样酪氨酸激酶3(FLT3)和异柠檬酸脱氢酶1或2的药物,大多局限于相关突变的存在。最近的研究不仅表明AML的特定突变会产生代谢脆弱性,而且还强调了与这些突变的抑制剂联合靶向代谢脆弱性的疗效。因此,阐明遗传分层、代谢依赖性和对这些脆弱性的特定抑制剂的反应之间的功能关系,对于确定更有效的治疗方案、了解耐药性机制和确定早期反应标志物,最终提高治愈的可能性至关重要。此外,肿瘤微环境中发生的代谢变化也被报道为治疗靶点。白血病干细胞(LSCs)的代谢特征不同,复发/难治性LSCs转向替代代谢途径,促进氧化磷酸化(OXPHOS),使其具有治疗耐药性。在这篇综述中,我们讨论了癌症代谢途径的作用,这些代谢途径有助于AML的代谢可塑性并赋予对标准治疗的耐药性;我们还强调了该领域在将这些重要发现转化为临床方面的最新进展,并讨论了支持代谢可塑性和与AML细胞相互作用的肿瘤微环境。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


