A photoswitchable fluorescent protein for hours-time-lapse and sub-second-resolved super-resolution imaging
IF 1.8
4区 工程技术
引用次数: 5
Abstract
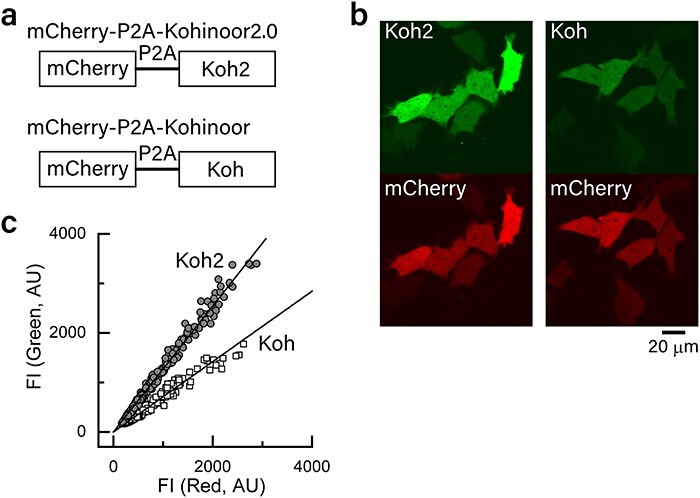
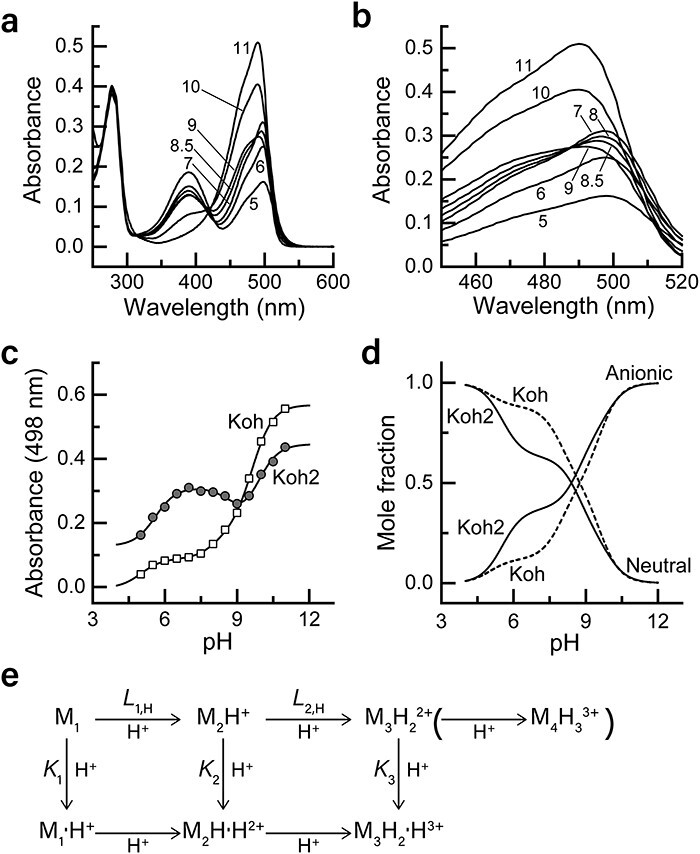
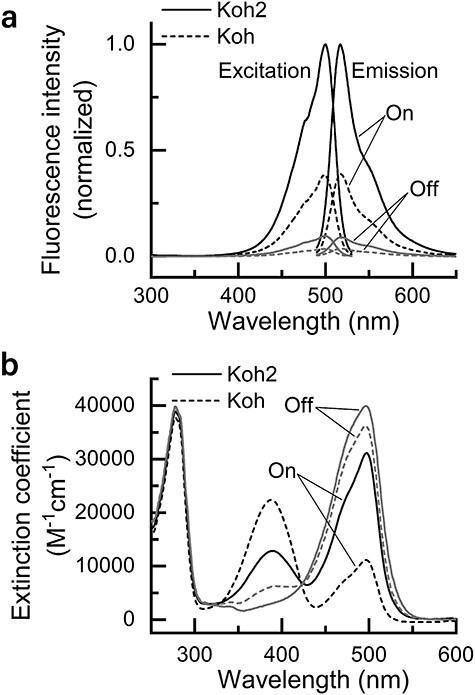
Reversibly photoswitchable fluorescent proteins (RSFPs) are a class of fluorescent proteins whose fluorescence can be turned on and off by light irradiation. RSFPs have become essential tools for super-resolution (SR) imaging. Because most SR imaging techniques require high-power-density illumination, mitigating phototoxicity in cells due to intense light irradiation has been a challenge. Although we previously developed an RSFP named Kohinoor to achieve SR imaging with low phototoxicity, the photoproperties were insufficient to move a step further to explore the cellular dynamics by SR imaging. Here, we show an improved version of RSFP, Kohinoor2.0, which is suitable for SR imaging of cellular processes. Kohinoor2.0 shows a 2.6-fold higher fluorescence intensity, 2.5-fold faster chromophore maturation and 1.5-fold faster off-switching than Kohinoor. The analysis of the pH dependence of the visible absorption band revealed that Kohinoor2.0 and Kohinoor were in equilibria among multiple fluorescently bright and dark states, with the mutations introduced into Kohinoor2.0 bringing about a higher stabilization of the fluorescently bright states compared to Kohinoor. Using Kohinoor2.0 with our SR imaging technique, super-resolution polarization demodulation/on-state polarization angle narrowing, we conducted 4-h time-lapse SR imaging of an actin filament network in mammalian cells with a total acquisition time of 480 s without a noticeable indication of phototoxicity. Furthermore, we demonstrated the SR imaging of mitochondria dynamics at a time resolution of 0.5 s, in which the fusion and fission processes were clearly visualized. Thus, Kohinoor2.0 is shown to be an invaluable RSFP for the SR imaging of cellular dynamics.
一种可光切换的荧光蛋白的小时延时和亚秒分辨率超分辨率成像
可逆光开关荧光蛋白(RSFP)是一类荧光蛋白,其荧光可以通过光照射打开和关闭。RSFP已经成为超分辨率(SR)成像的重要工具。由于大多数SR成像技术需要高功率密度照明,因此减轻细胞因强光照射而产生的光毒性一直是一个挑战。尽管我们之前开发了一种名为Kohinoor的RSFP,以实现低光毒性的SR成像,但光特性不足以进一步探索SR成像的细胞动力学。在这里,我们展示了RSFP的改进版本Kohinoor2.0,它适用于细胞过程的SR成像。Kohinoor2.0显示出比Kohinoor高2.6倍的荧光强度、2.5倍的发色团成熟速度和1.5倍的关断速度。对可见吸收带的pH依赖性的分析表明,Kohinoor 2.0和Kohinoor在多种荧光亮态和暗态之间处于平衡状态,与Kohinoo尔相比,引入Kohinoor2.0的突变带来了更高的荧光亮态稳定性。使用Kohinoor2.0和我们的SR成像技术,超分辨率偏振解调/状态偏振角变窄,我们对哺乳动物细胞中的肌动蛋白丝网络进行了4小时延时SR成像,总采集时间为480秒,没有明显的光毒性迹象。此外,我们展示了时间分辨率为0.5s的线粒体动力学SR成像,其中融合和裂变过程清晰可见。因此,Kohinoor2.0被证明是细胞动力学SR成像的宝贵RSFP。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microscopy
工程技术-显微镜技术
自引率
11.10%
发文量
0
审稿时长
>12 weeks
期刊介绍:
Microscopy, previously Journal of Electron Microscopy, promotes research combined with any type of microscopy techniques, applied in life and material sciences. Microscopy is the official journal of the Japanese Society of Microscopy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


