Structures and gating mechanism of human TRPM2
IF 44.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 109
Abstract
Transient receptor potential (TRP) melastatin 2 (TRPM2) is a cation channel associated with numerous diseases. It has a C-terminal NUDT9 homology (NUDT9H) domain responsible for binding adenosine diphosphate (ADP)–ribose (ADPR), and both ADPR and calcium (Ca2+) are required for TRPM2 activation. Here we report cryo–electron microscopy structures of human TRPM2 alone, with ADPR, and with ADPR and Ca2+. NUDT9H forms both intra- and intersubunit interactions with the N-terminal TRPM homology region (MHR1/2/3) in the apo state but undergoes conformational changes upon ADPR binding, resulting in rotation of MHR1/2 and disruption of the intersubunit interaction. The binding of Ca2+ further engages transmembrane helices and the conserved TRP helix to cause conformational changes at the MHR arm and the lower gating pore to potentiate channel opening. These findings explain the molecular mechanism of concerted TRPM2 gating by ADPR and Ca2+ and provide insights into the gating mechanism of other TRP channels.
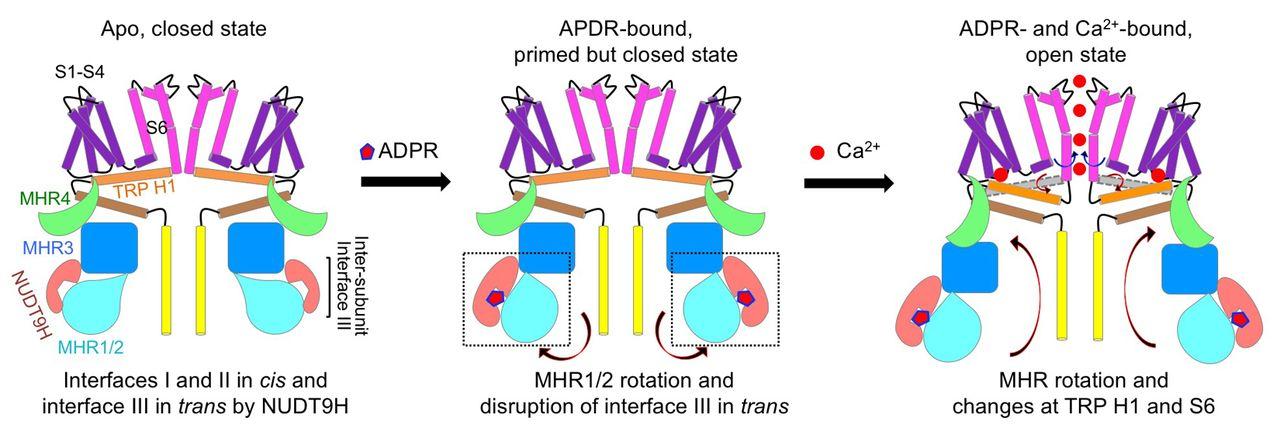
人类 TRPM2 的结构和门控机制
瞬时受体电位(TRP)美拉司他丁 2(TRPM2)是一种与多种疾病相关的阳离子通道。它有一个 C 端 NUDT9 同源(NUDT9H)结构域,负责结合二磷酸腺苷(ADP)-核糖(ADPR),TRPM2 的激活需要 ADPR 和钙(Ca2+)。在此,我们报告了人类 TRPM2 单独、与 ADPR 以及与 ADPR 和 Ca2+ 的冷冻电镜结构。在apo状态下,NUDT9H与N端TRPM同源区(MHR1/2/3)形成了亚基内和亚基间的相互作用,但在与ADPR结合时发生了构象变化,导致MHR1/2旋转并破坏了亚基间的相互作用。结合 Ca2+ 后,跨膜螺旋和保守的 TRP 螺旋进一步参与,导致 MHR 臂和较低的门孔发生构象变化,从而促进通道开放。这些发现解释了ADPR和Ca2+协同TRPM2门控的分子机制,并为其他TRP通道的门控机制提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


