HIF-1α Activates Mitophagy through BNIP3 to Inhibit IPEC-J2 Apoptosis under Heat Stress
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Hypoxia caused by heat stress (HS) exacerbates intestinal epithelial cell apoptosis, and mitochondrial dysfunction plays a central role in this process. Increased hypoxia-inducible factor 1α (HIF-1α) shows an obvious antiapoptotic effect under HS. Based on the relationship between HIF-1α, mitochondrial homeostasis and BNIP3, we hypothesize that HIF-1α promotes BNIP3-mediated mitophagy to protect intestinal epithelial cells from HS-induced apoptosis. The results showed that HS destroyed the physical barrier of porcine small intestinal mucosa (disorganized microvilli, disrupted tight junctions) in vivo, accompanied by mitochondrial structure damage. The ratio of LC3II/I (p < 0.001) in the duodenum, jejunum, and ileum increased in a time-dependent manner under 72 h HS, while P62 (p < 0.001) and the Bcl-2/BAX ratio (p < 0.001) decreased continuously. In vitro, proteomic sequencing of HS-treated IPEC-J2 confirmed a negative correlation between mitochondrial function and HS, indicating HS disrupted mitochondrial homeostasis. Further experiments showed that increased HIF-1α improved mitochondrial function and promoted mitophagy; the expression of HIF-1α (p < 0.001), BNIP3 (p < 0.001), p-AMPK/AMPK (p < 0.001), Parkin (p < 0.001), and LC3II/I (p < 0.001) was synchronously upregulated under HS, whereas P62 (p < 0.001) showed the opposite trend. Critically, inhibition of BNIP3 disrupted the effect of HIF-1α on mitophagy, indicating that HIF-1α promotes IPEC-J2 mitophagy through BNIP3 under HS. What was more, increased HIF-1α alleviated HS-induced cell apoptosis (p < 0.001), while inhibition of BNIP3 could block the antiapoptotic effect of HIF-1α. In conclusion, we first clarify HIF-1α alleviates HS-induced IPEC-J2 apoptosis through BNIP3-activated mitophagy, and this regulatory axis provides a molecular target for mitigating HS-induced intestinal damage in the swine industry.
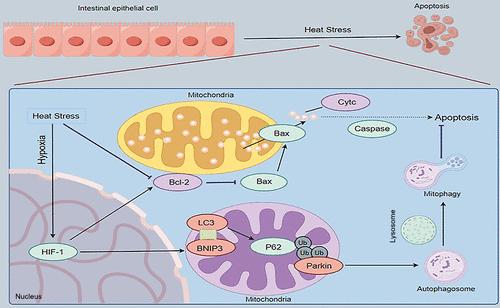
热应激下HIF-1α通过BNIP3激活线粒体自噬抑制IPEC-J2细胞凋亡
热应激引起的缺氧加剧了肠上皮细胞的凋亡,而线粒体功能障碍在这一过程中起着核心作用。缺氧诱导因子1α (HIF-1α)的升高显示出明显的抗凋亡作用。基于HIF-1α、线粒体稳态和BNIP3之间的关系,我们假设HIF-1α促进BNIP3介导的线粒体自噬,从而保护肠上皮细胞免受hs诱导的凋亡。结果表明,HS在体内破坏了猪小肠黏膜的物理屏障(微绒毛紊乱,紧密连接中断),并伴有线粒体结构损伤。72h HS下,十二指肠、空肠和回肠LC3II/I比值(p < 0.001)呈时间依赖性升高,P62 (p < 0.001)和Bcl-2/BAX比值(p < 0.001)持续降低。体外,HS处理的IPEC-J2的蛋白质组学测序证实了线粒体功能与HS之间的负相关,表明HS破坏了线粒体稳态。进一步实验表明,HIF-1α升高可改善线粒体功能,促进线粒体自噬;HS下HIF-1α (p < 0.001)、BNIP3 (p < 0.001)、p-AMPK/AMPK (p < 0.001)、Parkin (p < 0.001)、LC3II/I (p < 0.001)的表达同步上调,而P62 (p < 0.001)的表达则呈现相反的趋势。关键的是,抑制BNIP3破坏了HIF-1α对线粒体自噬的作用,表明HIF-1α在HS下通过BNIP3促进IPEC-J2的线粒体自噬。HIF-1α升高可减轻hs诱导的细胞凋亡(p < 0.001),而抑制BNIP3可阻断HIF-1α的抗凋亡作用。综上所述,我们首次阐明HIF-1α通过bnip3激活的线粒体自噬减轻hs诱导的IPEC-J2细胞凋亡,这一调控轴为减轻hs诱导的猪肠道损伤提供了分子靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


