Selective Capture and Addressable Release of Single Cells Enabled by Acoustic Resonator Integrated Serpentine Microchannel
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Single cell manipulation and analysis are crucial for understanding cellular heterogeneity, yet conventional microfluidic approaches suffer from poor selectivity, structural complexity, and low throughput. Here, we present an acoustofluidic-microstructure integrated platform combining a right triangle-shaped bulk acoustic wave (RTBAW) resonator with a serpentine microchannel. The platform enables selective capture (efficiency >90%) and addressable release (efficiency >90%) of single cells by leveraging acoustic streaming-induced hydrodynamic forces. Unlike existing methods relying on optical or electrical fields, our design eliminates thermal damage and ionic interference while achieving submillisecond response time and parallel processing of 4 cells per array unit. This simplified architecture reduces fabrication complexity and enhances throughput by directing untargeted cells through curved bypass channels. We validated the platform’s utility in live/dead cell sorting, demonstrating its potential for high-precision single-cell diagnostics, drug screening, and rare cell isolation.
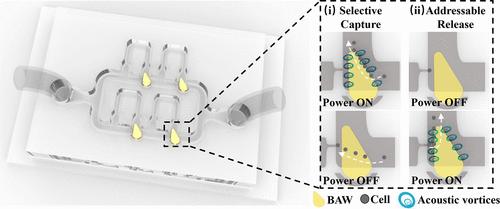
基于声学谐振器集成蛇形微通道的单细胞选择性捕获和可寻址释放
单细胞操作和分析对于理解细胞异质性至关重要,然而传统的微流体方法存在选择性差、结构复杂和低通量的问题。在这里,我们提出了一个结合了直角三角形体声波(RTBAW)谐振器和蛇形微通道的声流微结构集成平台。该平台通过利用声波流诱导的水动力,实现了单个细胞的选择性捕获(效率>;90%)和可寻址释放(效率>;90%)。与现有的依赖于光场或电场的方法不同,我们的设计消除了热损伤和离子干扰,同时实现了亚毫秒的响应时间和每个阵列单元4个单元的并行处理。这种简化的结构降低了制造的复杂性,并通过弯曲的旁路通道引导非靶向细胞提高了吞吐量。我们验证了该平台在活/死细胞分选中的实用性,展示了其在高精度单细胞诊断、药物筛选和罕见细胞分离方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


