Discovery of Thiazole-5-Carboxamide Derivatives Containing Diphenyl Ether Fragments as Potential Succinate Dehydrogenase Inhibitors
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Succinate dehydrogenase inhibitors (SDHIs) have become one of the fastest-growing categories in the fungicide market and are widely utilized for crop protection in agricultural production. Currently, guided by the imperative of cost reduction and efficiency enhancement, the replacement of biphenyl fragments in SDHIs with cost-effective diphenyl ether fragments has emerged as an innovative strategy for developing novel, highly efficient, and broad-spectrum fungicides. Based on the above structural features, 45 thiazole-5-carboxamide derivatives containing diphenyl ether fragments (potentially targeting fungal SDH) were designed and evaluated for their antifungal effects against Rhizoctonia solani, Sclerotinia sclerotiorum, Alternaria alternata, and Alternaria solani. Notably, the in vitro EC50 value of compound IIIe against R. solani was 0.009 mg/L, exhibiting significantly greater potency than thifluzamide (0.039 mg/L), boscalid (1.849 mg/L), fluxapyroxad (0.049 mg/L), and carboxin (0.146 mg/L), and proving comparable to that of the novel SDHIs fungicide flubeneteram (0.008 mg/L). Concurrently, compound IIIe demonstrated high efficacy in controlling rice sheath blight through detached leaf and pot experiments. Further investigations into fungal SDH inhibition, respiratory suppression, mitochondrial membrane potential detection, molecular docking, cell cytotoxicity, scanning electron microscopy, and transmission electron microscopy analysis confirmed the practical value of compound IIIe as a potential SDHI. The present results provide an indispensable complement for the structural optimization of antifungal leads to the targeting of SDH.
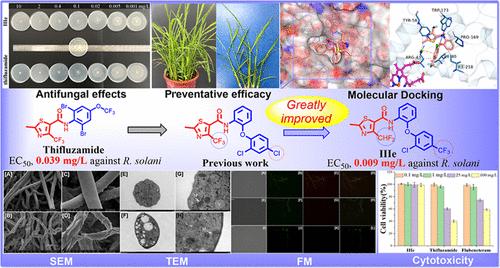
含有二苯基醚片段的噻唑-5-羧基酰胺衍生物作为琥珀酸脱氢酶抑制剂的发现
琥珀酸脱氢酶抑制剂(SDHIs)已成为杀菌剂市场发展最快的品种之一,广泛应用于农业生产中的作物保护。目前,在降低成本和提高效率的迫切需要的指导下,用具有成本效益的二苯基醚片段替代SDHIs中的联苯片段已成为开发新型、高效、广谱杀菌剂的创新策略。基于上述结构特征,设计了45种含二苯醚片段(可能靶向真菌SDH)的噻唑-5-羧基酰胺衍生物,并对其对茄根丝核菌、菌核菌、互花菌和茄疫病菌的抑菌效果进行了评价。值得注意的是,化合物IIIe对茄枯病菌的体外EC50值为0.009 mg/L,药效显著高于噻氟唑胺(0.039 mg/L)、boscalid (1.849 mg/L)、fluxapyroxad (0.049 mg/L)和carboxin (0.146 mg/L),与新型SDHIs杀菌剂氟苯醚(0.008 mg/L)相当。同时,通过离体叶片和盆栽试验,化合物IIIe对水稻纹枯病有较好的防治效果。进一步的真菌SDH抑制、呼吸抑制、线粒体膜电位检测、分子对接、细胞毒性、扫描电镜和透射电镜分析证实了化合物IIIe作为潜在SDH的实用价值。本研究结果为靶向SDH的抗真菌先导物结构优化提供了必要的补充。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


