Heat-Induced Protein Oxidation as an Impediment for Thermal Stability Measurements: A Case Study on Cytochrome c
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
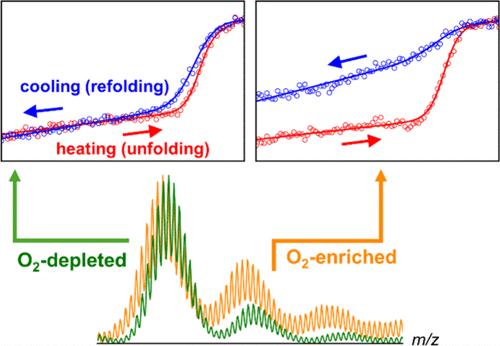
Thermal unfolding (“melting”) experiments are widely used for protein stability measurements. These assays probe spectroscopic properties of the protein while gradually increasing the solution temperature until unfolding is complete. Thermodynamic parameters are obtained from fits of the resulting profiles. Differential scanning calorimetry relies on similar concepts. Meaningful stability measurements require reversible conditions, where the protein fluctuates between its native and unfolded states (N ⇌ U), as governed by the temperature-dependent equilibrium constant. A simple reversibility test is to ensure that heating and cooling (unfolding and refolding) profiles are superimposable. Unfortunately, such tests are not commonly performed. Here, we focused on cytochrome c, one of the most widely used model proteins for folding studies. Surprisingly, thermal unfolding/refolding was found to be irreversible, even in the absence of aggregation. Using mass spectrometry (MS), we traced the origin of this irreversibility to oxidative side chain modifications that accumulate during thermal assays. By gradually altering the covalent composition of the protein, oxidation creates a scenario far from ideal N ⇌ U conditions, rendering the validity of fitted thermodynamic parameters questionable. Oxidation at Tyr, Trp, and Met residues was promoted by dissolved O2. It appears that the role of oxidation as an impediment for protein stability assays has been overlooked in the past. While the use of deoxygenated solutions represents a partial remedy, it is hoped that better oxidation suppression strategies will be developed in the future. In any case, it is advisable to perform MS measurements alongside thermal protein stability experiments to ensure that problems related to oxidation-mediated irreversibility are properly recognized.
热诱导的蛋白质氧化作为热稳定性测量的障碍:细胞色素c的案例研究
热展开(“融化”)实验被广泛用于蛋白质稳定性测量。这些实验探测蛋白质的光谱性质,同时逐渐提高溶液温度,直到展开完成。热力学参数由所得剖面的拟合得到。差示扫描量热法依赖于类似的概念。有意义的稳定性测量需要可逆条件,其中蛋白质在其天然状态和未折叠状态(N + U)之间波动,由温度依赖的平衡常数控制。一个简单的可逆性测试是确保加热和冷却(展开和折叠)型材是重叠的。不幸的是,这样的测试并不常见。在这里,我们专注于细胞色素c,折叠研究中最广泛使用的模型蛋白之一。令人惊讶的是,热展开/再折叠被发现是不可逆的,即使在没有聚集的情况下。使用质谱法(MS),我们追溯了这种不可逆性的起源,氧化侧链修饰在热分析过程中积累。通过逐渐改变蛋白质的共价组成,氧化产生了一个远离理想N + U条件的场景,使得拟合的热力学参数的有效性受到质疑。溶解的氧促进了Tyr、Trp和Met残基的氧化。在过去,氧化作为蛋白质稳定性测定的障碍的作用似乎被忽视了。虽然脱氧溶液的使用是部分补救措施,但希望将来能开发出更好的氧化抑制策略。在任何情况下,建议在热蛋白稳定性实验的同时进行质谱测量,以确保正确识别与氧化介导的不可逆性相关的问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


