Antibody-Free Inline Dual-Retention Nano-WCX-μSPE-MS/MS for Quantification of Intact Parathyroid Hormone and Antagonistic Fragments in Clinical Serum
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
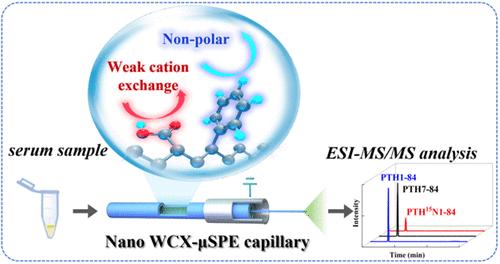
Parathyroid hormone (PTH), an 84-amino acid polypeptide critical for calcium/phosphorus homeostasis, exhibits significant analytical challenges in clinical quantification, particularly in chronic kidney disease (CKD) patients due to its low circulating concentrations, biological matrix complexity, and interference from circulating PTH fragments. Traditional immunoassays may either overestimate the PTH level due to cross-reactivity with the PTH 7–84 or fail to quantify the antagonistic fragment. Highly sensitive and selective mass spectrometry (MS)-based methods could be used as the reference measurement procedure (RMP) for PTH assays, once the limitations of quantification thresholds and antibody-dependent workflows are addressed. Here, we introduce the first inline weak cation-exchange microsolid-phase extraction-tandem mass spectrometry (WCX-μSPE-MS/MS) method for simultaneous quantification of PTH 1–84 and its antagonist PTH 7–84 in clinical serum samples. A carboxyl-functionalized polystyrene nanosphere-modified capillary μSPE column with dual weak cation exchange (WCX) and nonpolar retention mechanisms is integrated with electrospray ionization-MS/MS, enabling online purification, enrichment, and detection in a single analysis. The nanoliter-scale capillary extraction can minimize matrix effects and improve sensitivity while greatly eliminating offline sample transfer steps. A limit of detection (LOD) as low as 6.0 pg/mL (PTH 1–84) or 9.0 pg/mL (PTH 7–84) is achieved, with relative standard deviations less than 10%. Our antibody-free strategy greatly reduces solvent/sample consumption, avoids manual errors, and has been successfully applied for accurate quantification of both PTH 1–84 and PTH 7–84 in clinical serum, demonstrating great potential to serve as an RMP for PTH assays, enhancing diagnostic accuracy for hyperparathyroidism, CKD, and renal bone diseases.
无抗体在线双保留纳米wcx -μSPE-MS/MS定量临床血清中完整甲状旁腺激素和拮抗片段
甲状旁腺激素(PTH)是一种由84个氨基酸组成的多肽,对钙/磷稳态至关重要,由于其低循环浓度、生物基质复杂性和循环PTH片段的干扰,在临床定量分析中表现出重大挑战,特别是在慢性肾病(CKD)患者中。由于与PTH 7-84的交叉反应性,传统的免疫测定可能会高估PTH水平,或者无法量化拮抗片段。一旦解决了定量阈值和抗体依赖工作流程的限制,基于高灵敏度和选择性质谱(MS)的方法可作为PTH检测的参考测量程序(RMP)。本文首次建立了在线弱阳离子交换微固相萃取-串联质谱(WCX-μSPE-MS/MS)同时定量临床血清样品中PTH 1-84及其拮抗剂PTH 7-84的方法。一种具有双弱阳离子交换(WCX)和非极性保留机制的羧基功能化聚苯乙烯纳米球修饰毛细管μSPE柱与电喷雾电离-质谱联用(MS/MS)相结合,实现了一次在线纯化、富集和检测。纳米级毛细管萃取可以最大限度地减少基质效应,提高灵敏度,同时大大消除了离线样品转移步骤。检测限(LOD)低至6.0 pg/mL (PTH 1-84)或9.0 pg/mL (PTH 7-84),相对标准偏差小于10%。我们的无抗体策略大大减少了溶剂/样品消耗,避免了人工错误,并已成功应用于临床血清中PTH 1-84和PTH 7-84的准确定量,显示出作为PTH检测的RMP的巨大潜力,提高了甲状旁腺功能亢进、CKD和肾骨病的诊断准确性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


