Formic Acid Pretreatment Enhances Untargeted Serum and Plasma Metabolomics
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Untargeted metabolic profiling of plasma and serum by liquid chromatography–mass spectrometry (LC-MS) is becoming increasingly important in clinical and translational research; however, sample preparation protocols can have a significant impact on study outcomes, and there is currently a lack of standardized approaches. In this study we demonstrate that pretreatment of serum and plasma samples with 1% formic acid (FA, v/v) prior to acetonitrile (MeCN)-induced protein precipitation significantly enhances analytical performance in untargeted metabolomics using reversed-phase liquid chromatography (RPLC)-MS. We show an increase in sample preparation reproducibility and signal intensity across both positive and negative ionization modes. In two independent serum cohorts (OPTIMA and VITACOG), FA-based extraction improved multivariate modeling (orthogonal partial least-squares discriminant analysis, OPLS-DA), with consistently higher classification accuracy, sensitivity, and specificity, alongside reduced variability and increased fold-changes in discriminatory compound-features. We investigated factors potentially involved in the enhanced performance and observed outcomes consistent with the disruption of noncovalent protein–metabolite interactions and the stabilization of labile species. We found no correlation with either protein depletion or differential adduct formation. The results were also not attributable to lowering pH after metabolite extraction. In summary, we demonstrate that FA pretreatment of plasma and serum, prior to protein precipitation, significantly improves sample reproducibility and detection sensitivity in untargeted RPLC-MS metabolomics. This optimized sample preparation strategy offers clear advantages for clinical and translational metabolomics, with the potential to enhance biomarker discovery and metabolic phenotyping.
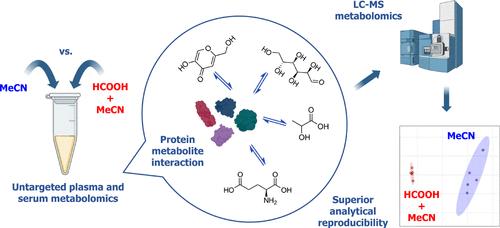
甲酸预处理增强非靶向血清和血浆代谢组学
液相色谱-质谱法(LC-MS)对血浆和血清的非靶向代谢谱分析在临床和转化研究中变得越来越重要;然而,样品制备方案可能对研究结果产生重大影响,目前缺乏标准化的方法。在这项研究中,我们证明了在乙腈(MeCN)诱导的蛋白质沉淀之前,用1%甲酸(FA, v/v)预处理血清和血浆样品,可以显著提高反相液相色谱(RPLC)-MS在非靶向代谢组学中的分析性能。我们展示了样品制备的可重复性和信号强度在正负电离模式的增加。在两个独立的血清队列(OPTIMA和VITACOG)中,基于fa的提取改进了多变量建模(正交偏最小二乘判别分析,OPLS-DA),具有更高的分类准确性、灵敏度和特异性,同时减少了可变性,增加了鉴别化合物特征的倍数变化。我们研究了可能与提高性能有关的因素,并观察到与非共价蛋白-代谢物相互作用的破坏和不稳定物种的稳定一致的结果。我们没有发现蛋白质消耗或差异加合物形成的相关性。结果也不能归因于代谢物提取后pH值的降低。总之,我们证明,在蛋白质沉淀之前,对血浆和血清进行FA预处理,显著提高了非靶向RPLC-MS代谢组学的样品重复性和检测灵敏度。这种优化的样品制备策略为临床和转化代谢组学提供了明显的优势,具有增强生物标志物发现和代谢表型的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


