The superiority of non-gastrointestinal hydrolysis in preparing egg white-derived peptides for orally colitis mitigation: Transepithelial transport and microbiota regulation
IF 8
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Oral food protein-derived peptides for healthcare have attracted increasing attention. However, most animal proteins (e.g., egg white) are highly in vivo digestible into peptides without additional hydrolysis treatment, which poses evident challenges to consumer choices and applications of peptide-based functional food. Herein, egg white-derived peptides prepared by alcalase hydrolysis (APH) and in vitro simulated digestion (SGDP) were compared to investigate the superiority of non-gastrointestinal hydrolysis in preparing peptides. Results indicated that the small peptide (<1 kDa) ratio, proteolysis degree, and sequence diversity of APH were double that of SGDP owing to broader cleavage sites. APH with C-terminal hydrophobic uncharged amino acid residues and higher positivity exhibited admirable advantages in transepithelial transport, contributing to better bioactivities (e.g., antioxidant and anti-inflammatory) and amino acid supplementation. Moreover, APH preferentially enriched mucus- and short-chain fatty acid-associated probiotics, thereby achieving preferable inflammation relief and microbiota restoration. Overall, this study offers an integrated theoretical basis for the superiority of non-gastrointestinal hydrolysis in preparing egg white-derived peptides for healthcare (e.g., colitis relief). Meanwhile, these findings would be enlightening to the development of enzymatic combination strategies and peptide-based ingredients.
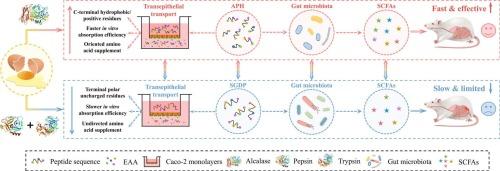
非胃肠道水解制备蛋清衍生肽用于口服结肠炎的优势:经上皮转运和微生物群调节
用于保健的口服食品蛋白衍生肽越来越受到人们的关注。然而,大多数动物蛋白(如蛋清)在体内可高度消化为多肽,无需额外的水解处理,这对消费者选择和应用基于多肽的功能食品提出了明显的挑战。本研究比较了alcalase水解法(APH)和体外模拟消化法(SGDP)制备的蛋清肽,探讨了非胃肠道水解法制备肽的优越性。结果表明,APH的小肽(<1 kDa)比例、蛋白水解程度和序列多样性是SGDP的两倍,因为它的裂解位点更宽。具有c端疏水不带电氨基酸残基和较高阳性的APH在经上皮运输中表现出令人称赞的优势,有助于更好的生物活性(如抗氧化和抗炎)和氨基酸补充。此外,APH优先富集黏液和短链脂肪酸相关益生菌,从而达到较好的炎症缓解和微生物群恢复效果。总的来说,本研究为非胃肠道水解制备蛋清衍生肽的优越性提供了一个完整的理论基础,用于医疗保健(例如,缓解结肠炎)。同时,这些发现对酶结合策略和肽类成分的开发具有一定的启示意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


